INSTANT EGGHEAD
GUIDE: PHYSICS
ALSO BY BRIAN CLEGG
Before the Big Bang: The Prehistory of Our Universe
A Brief History of Infinity
The First Scientist: A Life of Roger Bacon
Light Years: An Exploration of Mankinds
Enduring Fascination with Light
Upgrade Me: Our Amazing Journey to Human 2.0
BRIAN CLEGG AND
SCIENTIFIC AMERICAN
INSTANT
EGGHEAD


ST. MARTINS GRIFFIN  NEW YORK
NEW YORK
INSTANT EGGHEAD GUIDE: PHYSICS . Copyright 2009 by Scientific American. All rights reserved. Printed in the United States of America. For information, address St. Martins Press, 175 Fifth Avenue, New York, N.Y. 10010.
www.stmartins.com
Library of Congress Cataloging-in-Publication Data
Clegg, Brian.
Instant egghead guide. Physics / Brian Clegg and Scientific American.1st ed.
p. cm.
ISBN 978-0-312-59210-3
1. Quantum theoryPopular works.
I. Scientific American, inc. II. Title. III. Title: Physics.
QC174.12.C546 2009
530dc22
2009017043
First Edition: November 2009
10 9 8 7 6 5 4 3 2 1
CONTENTS
CHAPTER ONE
MATTER
CHAPTER TWO
QUANTUM THEORY
CHAPTER THREE
LIGHT
CHAPTER FOUR
RELATIVITY
CHAPTER FIVE
FORCES
CHAPTER SIX
ENERGY
INSTANT EGGHEAD
GUIDE: PHYSICS
CHAPTER

STUFF
THE BASICS
Physics, the topic of this book, is the fundamental science. The great physicist Ernest Rutherford once said, All science is either physics or stamp collecting. He meant that most other science at the time was about collecting and categorizing information. Physics explained how the universe works.
In this section, the focus is stuff. Whats this bookor youmade of? Is everything made of the same kind of stuff? Why is some stuff hard and some fluid? How do you turn one kind of stuff into another?
To the modern mind, stuff is obviously made up of atoms, assembled out of tiny components like a vast LEGO structure. Yet this isnt obvious. Look at a glass of water. Both the glass and the water inside seem to be continuous substance, quite unlike anything were familiar with thats made up of small components. To understand matter we have to go beyond the obvious, to see in our minds what we cant experience with our senses, and thats part of the fun of physics.
ON THE FRONTIER
An atom is the single smallest particle of an element. It is as small as you can get and still have a bit of that substance. Molecules contain more than one atom, joined together. This can be a pure element. For example, a molecule of oxygen contains two oxygen atoms, joined together. But it can also contain several elements, whether its a simple molecule such as sodium chloride or the immensely long DNA chains that make up the complex molecules of life.
COCKTAIL PARTY TIDBITS | 
|
 One ancient Greek theory on matter suggested that you could cut stuff up into smaller and smaller pieces until eventually you could cut no more. What was left was uncuttablein Greek, a-tomosatoms.
One ancient Greek theory on matter suggested that you could cut stuff up into smaller and smaller pieces until eventually you could cut no more. What was left was uncuttablein Greek, a-tomosatoms.
 If you could squeeze all the matter in your body together, removing the empty space, it would pack into a cube less than a thousandth of an inch per side.
If you could squeeze all the matter in your body together, removing the empty space, it would pack into a cube less than a thousandth of an inch per side.
BROWNIAN MOTION
THE BASICS
Atoms are like small children: they are never entirely still. Its a remarkable contrast between the visible world and the world of the very small. Look at a glass of water. That water appears to be motionless, yet within the liquid the water molecules are frantically dancing.
In 1827 a Scottish botanist called Robert Brown was studying pollen grains of an evening primrose plant, suspended in a drop of water under a microscope. The tiny specks of pollen jumped about, constantly in motion.
There seemed to be no order to the motion, no rules for the way they moved. Instead the pollen grains dancing was chaotic. This jerky dance was named Brownian motion, but remained little more than an oddity until Albert Einstein linked it to the behavior of atoms.
ON THE FRONTIER
Einstein produced three great papers in 1905. His works on relativity and the photoelectric effect get the glory, but his third paper on Brownian motion was just as significant. Until that time, the concept of atoms was entirely theoretical. But Einstein showed that the dance of the pollen grains was caused by the random impact of billions of water molecules. Einstein used Brownian motion to show that the liquid the grains floated in was composed of many billions of gyrating molecules.
COCKTAIL PARTY TIDBITS | 
|
 When Robert Brown first saw Brownian motion he suspected it was the life source of a plant in action. It was only when he tried stone dust and soot and found the same effect with particles that were never alive that he confirmed that the size of the pollen grains was responsible for their motion.
When Robert Brown first saw Brownian motion he suspected it was the life source of a plant in action. It was only when he tried stone dust and soot and found the same effect with particles that were never alive that he confirmed that the size of the pollen grains was responsible for their motion.
 It wasnt until 1912 that French physicist Jean Perrin firmly established the existence of atoms. Until then, many scientists denied they existed.
It wasnt until 1912 that French physicist Jean Perrin firmly established the existence of atoms. Until then, many scientists denied they existed.
ATOMIC STRUCTURE
THE BASICS
It was surprisingly soon after finding proof that atoms existed that the name atom proved to be inaccurate. Even as Brownian motion was showing atoms and molecules to be real, it was becoming obvious that atoms werent uncuttable.
A British scientist, J. J. Thomson, discovered in 1895 that there was a negatively charged particle within the atom, which would be named the electron. He imagined atoms were like a pudding with raisins spread through it. The raisins were the electrons and the rest of the pudding was positively charged, balancing this out so the atom had no charge itself.
ON THE FRONTIER
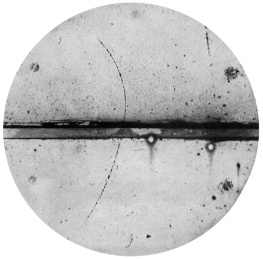
Thomsons pudding model was blasted apart by New Zealand scientist Ernest Rutherford. Rutherford had the idea of firing other particles into an atom and seeing how they reacted, like throwing a ball repeatedly at an invisible object and working out its structure by seeing how the ball bounces off. The ball he used was an alpha particle, the nucleus of the helium atom. These particles could be detected when they hit screens painted with fluorescent material. If the atom had been as Thomson imagined, the powerful alpha particles should plow through. Most did, but occasionally one bounced back. This unexpected result made Rutherford realize that atoms have a small, dense, positively charged core to deflect the alpha particles. He established the familiar idea of the atom being like a solar system with a positive nucleus at the center and negative electrons floating around it.


















 NEW YORK
NEW YORK

 One ancient Greek theory on matter suggested that you could cut stuff up into smaller and smaller pieces until eventually you could cut no more. What was left was uncuttablein Greek, a-tomosatoms.
One ancient Greek theory on matter suggested that you could cut stuff up into smaller and smaller pieces until eventually you could cut no more. What was left was uncuttablein Greek, a-tomosatoms.