Also in McGraw-Hills 500 Questions SeriesMcGraw-Hills 500 American Government Questions: Ace Your College Exams
McGraw-Hills 500 College Algebra and Trigonometry Questions: Ace Your College Exams
McGraw-Hills 500 College Biology Questions: Ace Your College Exams
McGraw-Hills 500 College Calculus Questions: Ace Your College Exams
McGraw-Hills 500 College Chemistry Questions: Ace Your College Exams
McGraw-Hills 500 College Physics Questions: Ace Your College Exams
McGraw-Hills 500 Differential Equations Questions: Ace Your College Exams
McGraw-Hills 500 European History Questions: Ace Your College Exams
McGraw-Hills 500 French Questions: Ace Your College Exams
McGraw-Hills 500 Linear Algebra Questions: Ace Your College Exams
McGraw-Hills 500 Macroeconomics Questions: Ace Your College Exams
McGraw-Hills 500 Microeconomics Questions: Ace Your College Exams
McGraw-Hills 500 Organic Chemistry Questions: Ace Your College Exams
McGraw-Hills 500 Philosophy Questions: Ace Your College Exams
McGraw-Hills 500 Physical Chemistry Questions: Ace Your College Exams
McGraw-Hills 500 Precalculus Questions: Ace Your College Exams
McGraw-Hills 500 Psychology Questions: Ace Your College Exams
McGraw-Hills 500 Spanish Questions: Ace Your College Exams
McGraw-Hills 500 U.S. History Questions, Volume 1: Ace Your College Exams
McGraw-Hills 500 U.S. History Questions, Volume 2: Ace Your College Exams
McGraw-Hills 500 World History Questions, Volume 1: Ace Your College Exams
McGraw-Hills 500 World History Questions, Volume 2: Ace Your College Exams
McGraw-Hills 500 MCAT Biology Questions to Know by Test Day
McGraw-Hills 500 MCAT General Chemistry Questions to Know by Test Day
McGraw-Hills 500 MCAT Physics Questions to Know by Test Day
 David E. Goldberg, PhD, was formerly professor of chemistry and department chairman at Brooklyn College City University of New York. He is the author of widely used chemistry textbooks as well as Schaums Outline of Beginning Chemistry. Copyright 2013 by McGraw-Hill.
David E. Goldberg, PhD, was formerly professor of chemistry and department chairman at Brooklyn College City University of New York. He is the author of widely used chemistry textbooks as well as Schaums Outline of Beginning Chemistry. Copyright 2013 by McGraw-Hill.
All rights reserved. Except as permitted under the United States Copyright Act of 1976, no part of this publication may be reproduced or distributed in any form or by any means, or stored in a database or retrieval system, without the prior written permission of the publisher. ISBN: 978-0-07-179701-6
MHID: 0-07-179701-7 The material in this eBook also appears in the print version of this title: ISBN: 978-0-07-179700-9, MHID: 0-07-179700-9. All trademarks are trademarks of their respective owners. Rather than put a trademark symbol after every occurrence of a trademarked name, we use names in an editorial fashion only, and to the benefit of the trademark owner, with no intention of infringement of the trademark. Where such designations appear in this book, they have been printed with initial caps.
McGraw-Hill eBooks are available at special quantity discounts to use as premiums and sales promotions, or for use in corporate training programs. To contact a representative please e-mail us at bulksales@mcgraw-hill.com. TERMS OF USE This is a copyrighted work and The McGraw-Hill Companies, Inc. (McGraw-Hill) and its licensors reserve all rights in and to the work. Use of this work is subject to these terms. Except as permitted under the Copyright Act of 1976 and the right to store and retrieve one copy of the work, you may not decompile, disassemble, reverse engineer, reproduce, modify, create derivative works based upon, transmit, distribute, disseminate, sell, publish or sublicense the work or any part of it without McGraw-Hills prior consent.
You may use the work for your own noncommercial and personal use; any other use of the work is strictly prohibited. Your right to use the work may be terminated if you fail to comply with these terms. THE WORK IS PROVIDED AS IS. McGRAW-HILL AND ITS LICENSORS MAKE NO GUARANTEES OR WARRANTIES AS TO THE ACCURACY, ADEQUACY OR COMPLETENESS OF OR RESULTS TO BE OBTAINED FROM USING THE WORK, INCLUDING ANY INFORMATION THAT CAN BE ACCESSED THROUGH THE WORK VIA HYPERLINK OR OTHERWISE, AND EXPRESSLY DISCLAIM ANY WARRANTY, EXPRESS OR IMPLIED, INCLUDING BUT NOT LIMITED TO IMPLIED WARRANTIES OF MERCHANTABILITY OR FITNESS FOR A PARTICULAR PURPOSE. McGraw-Hill and its licensors do not warrant or guarantee that the functions contained in the work will meet your requirements or that its operation will be uninterrupted or error free. Neither McGraw-Hill nor its licensors shall be liable to you or anyone else for any inaccuracy, error or omission, regardless of cause, in the work or for any damages resulting therefrom.
McGraw-Hill has no responsibility for the content of any information accessed through the work. Under no circumstances shall McGraw-Hill and/or its licensors be liable for any indirect, incidental, special, punitive, consequential or similar damages that result from the use of or inability to use the work, even if any of them has been advised of the possibility of such damages. This limitation of liability shall apply to any claim or cause whatsoever whether such claim or cause arises in contract, tort or otherwise.
CONTENTS
Questions 113 Questions 1430 Questions 3150 Questions 5175 Questions 7687 Questions 88111 Questions 112137 Questions 138154 Questions 155178 Questions 179198 Questions 199213 Questions 214245 Questions 246282 Questions 283299 Questions 300316 Questions 317330 Questions 331365 Questions 366392 Questions 393410 Questions 411438 Questions 439453 Questions 454481 Questions 482500
INTRODUCTION
Youve taken a big step toward success in chemistry by purchasing
McGraw-Hills 500 College Chemistry Questions. We are here to help you take the next step and score high on your first-year exams! This book gives you 500 exam-style questions that cover all the most essential course material. Each question is clearly explained in the answer key.
The questions will give you valuable independent practice to supplement your regular textbook and the ground you have already covered in your class. This book and the others in the series were written by experienced teachers who know the subject inside and out and can identify crucial information as well as the kinds of questions that are most likely to appear on exams. You might be the kind of student who needs extra study before the exam for a final review. Or you might be the kind of student who puts off preparing until the last minute before the test. No matter what your preparation style, you will benefit from reviewing these 500 questions, which closely parallel the content and degree of difficulty of the questions on actual exams. These questions and the explanations in the answer key are the ideal last-minute study tool.
If you practice with all the questions and answers in this book, we are certain you will build the skills and confidence needed to excel on your exams. Editors of McGraw-Hill Education
CHAPTER 1
Measurement
Metric System
. Evaluate:
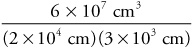 (A)
(A) Express 4.23 m in kilometers, in centimeters, and in millimeters. (B) Express 29.66 mm in centimeters and in meters.
(A) How many cubic centimeters are in 1 m3? (B) How many liters are in 1 m3? (C) How many cubic centimeters are in 1 L? Find the capacity in liters of a tank 0.50 m long, 20 cm wide, and 25 mm deep. Convert (A) 3.00 102 g to milligrams (B) 1.90 102 cm3 to liters (C) 6.21 km to millimeters (D) 3.33 mL to cubic centimeters (E) 9.70 106 mg to kilograms (F) 2.22 103 L to milliliters (G) 6.55 g/L to grams per milliliter (H) 4.18 kg/L to grams per milliliter Perform the following calculations. (A) (3.00 102 cm) + (7.50 101 m) (B) (0.200 m) (1.00 104 km) (3.00 102 mm)
Next page



















 David E. Goldberg, PhD, was formerly professor of chemistry and department chairman at Brooklyn College City University of New York. He is the author of widely used chemistry textbooks as well as Schaums Outline of Beginning Chemistry. Copyright 2013 by McGraw-Hill.
David E. Goldberg, PhD, was formerly professor of chemistry and department chairman at Brooklyn College City University of New York. He is the author of widely used chemistry textbooks as well as Schaums Outline of Beginning Chemistry. Copyright 2013 by McGraw-Hill.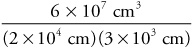 (A) Express 4.23 m in kilometers, in centimeters, and in millimeters. (B) Express 29.66 mm in centimeters and in meters. (A) How many cubic centimeters are in 1 m3? (B) How many liters are in 1 m3? (C) How many cubic centimeters are in 1 L? Find the capacity in liters of a tank 0.50 m long, 20 cm wide, and 25 mm deep. Convert (A) 3.00 102 g to milligrams (B) 1.90 102 cm3 to liters (C) 6.21 km to millimeters (D) 3.33 mL to cubic centimeters (E) 9.70 106 mg to kilograms (F) 2.22 103 L to milliliters (G) 6.55 g/L to grams per milliliter (H) 4.18 kg/L to grams per milliliter Perform the following calculations. (A) (3.00 102 cm) + (7.50 101 m) (B) (0.200 m) (1.00 104 km) (3.00 102 mm)
(A) Express 4.23 m in kilometers, in centimeters, and in millimeters. (B) Express 29.66 mm in centimeters and in meters. (A) How many cubic centimeters are in 1 m3? (B) How many liters are in 1 m3? (C) How many cubic centimeters are in 1 L? Find the capacity in liters of a tank 0.50 m long, 20 cm wide, and 25 mm deep. Convert (A) 3.00 102 g to milligrams (B) 1.90 102 cm3 to liters (C) 6.21 km to millimeters (D) 3.33 mL to cubic centimeters (E) 9.70 106 mg to kilograms (F) 2.22 103 L to milliliters (G) 6.55 g/L to grams per milliliter (H) 4.18 kg/L to grams per milliliter Perform the following calculations. (A) (3.00 102 cm) + (7.50 101 m) (B) (0.200 m) (1.00 104 km) (3.00 102 mm)