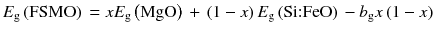1.1 Introduction
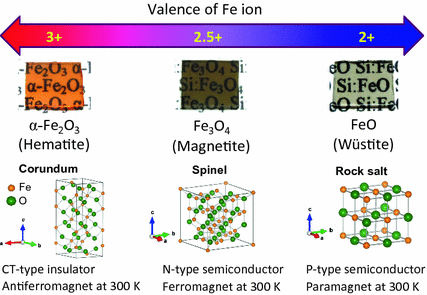
The challenge of developing a new material technology that can solve increasingly serious problems on a global scale, pertaining to the environment, energy, and resources, is being pursued actively. It is against such a backdrop that iron oxide , which is an inexhaustible resource that is nontoxic and offers a superior environmental affinity, is drawing much attention. Iron oxide has been used in a various devices, such as recording media to communication equipment. It is a representative magnetic material that has been supporting the development of modern industry from the ground up. The aspects of iron oxide responsible for its wide applicability are its high magnetic transition temperature (500 C and higher) and superior chemical stability. Most iron oxide materials currently being used in practical applications contain iron in the stable +3 valence state. Their localized spin allows for strong bond formation via oxygen ions (i.e. superexchange interaction) to achieve stable magnetic structure and magnetic field responsiveness, as well as high insulation, all of which are essential for practical applications. Transition metal oxides that contain iron oxide, on the other hand, feature an attractive characteristic of their electrical, magnetic, and optical properties varying significantly according to the valence of metal ions []. In iron oxides , the control of the valence states of Fe ions has been particularly difficult, and this has been a significant barrier for the progress of research and development in the application of new functional materials. This chapter describes the attempts made to control the valence of iron oxide using the film growth technique based on pulsed laser deposition (PLD). A variety of iron oxide thin films fabricated using these methods are then explored for their potentials as functional materials.
1.2 FeO: Transparent P-Type Oxide Semiconductor
Iron oxides are known to exhibit a wide range of physical properties and crystal structures. For example, multifunctional bismuth ferrite (BiFeO3) has been attracted considerable owing to its numerous promising applications in multiferroic and photovoltaic devices [].
Fig. 1.1
Basic iron oxides with different valence state of Fe ions in the electron conduction path and their physical properties. Images and crystal structures of these iron oxides are also shown
In this section, we focus on FeO thin films with a rock-salt crystal structure. FeO has attracted scientific interest over a long period because of its importance as a possible chemical component of the Earths core. Furthermore, FeO is currently a subject of intense investigation in a wide variety of research fields such as spintronics and chemical engineering []. Therefore, the extraction of the spinel phase may be suppressed by the substitution of Si in the film. In addition, the substitution of Si4+ in FeO enhances the [Fe2+]/[Fe3+] ionic ratio owing to charge neutrality, which is expected to improve the antioxidant properties of the films.
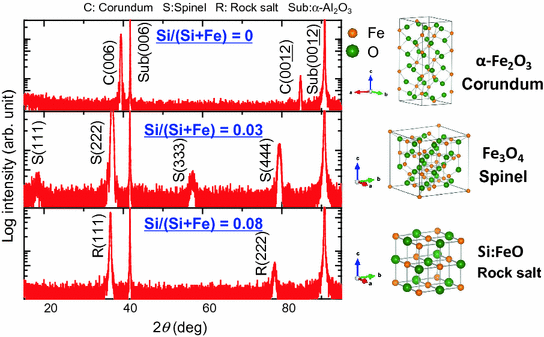
XRD patterns of the films prepared using PLD technique with Fe2O3SiO2 mixture target [] direction. In contrast to the FeO samples used in previous studies, no impurity phases such as Fe or Fe3O4 were observed in the XRD pattern of the Si:FeO film.
Fig. 1.2
XRD patterns for the films grown on -Al2O3 (001) by PLD using targets with Si/(Si + Fe) atomic rations of 0.00, 0.03, and 0.08. The second XRD pattern from the top is for the film annealed in air at 800 C for 3 h. C, S, and R denote the XRD peaks of the corundum, spinel, and rock-salt phases, respectively. The crystal structures of the films are illustrated next to the XRD patterns
Figure ]
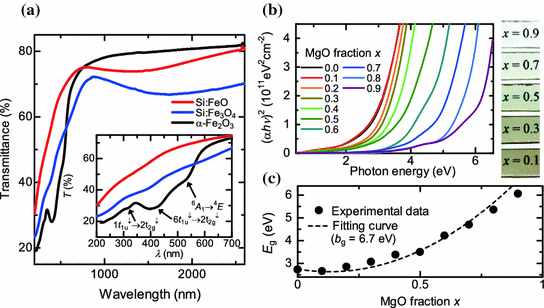
where b g is the so-called bowing parameter and E g(FSMO), E g(MgO), and E g(Si:FeO) are the bandgaps of FSMO, MgO, and Si:FeO, respectively. The dependence of the optical bandgap on the MgO fraction is well fitted with b g = 6.7 eV, as shown in Fig. ] and hence may be responsible for the large bandgap bowing in the FSMO films.
Fig. 1.3
a Optical transmittance spectra of the -Fe2O3, Si:Fe3O4, and Si:FeO films. Inset shows the enlarged spectra. b Tauc plots for MgO-Si:FeO solid solution thin films ( left ) and images of the films ( x = 0.1, 0.3, 0.5, and 0.9; right ). c Bandgap energy of MgOSi:FeO solid solution thin films as a function of MgO fraction x
Figure c), and hence presumably reflects the bowing of the valence band. In general, the limited conductivity of p -type wide-gap oxides has been the main difficulty in electrode and hole injection applications. The conductivity of the FSMO films is 0.17.8 S/cm at 300 K without the intentional doping of acceptors, which is significantly higher than that of most nondoped p -type wide-gap semiconducting oxides. In addition, note that the elements constituting the film (i.e., Fe, Si, and Mg) are abundant in the Earths crust, which means that the FSMO film is feasible for application in low cost and environmentally-friendly electronics.