Quantum physics is thought, without doubt, to be one of the greatest intellectual achievements of the 20th century. Its history began at the turn from the 19th to the 20th century. But we are confronted with its profound scientific, technological and philosophical implications today even more than ever. Not only in scientific original papers and text books but also in popular science literature and fiction more and more frequently book titles appear which contain terms as quantum theory, quantum mechanics, quantum physics, quantum world or quantum entrainment etc. Sometimes these titles are abused to supply quite questionable and esoteric treatises with a quasi-scientific background. What, therefore, is it all about with this field of quantum physics, which plays a central role in the education of physicists and, hopefully soon, also of chemists, biologists and engineers.
1.1 General and Historical Remarks
Isaac Newton created, more than 300 years ago, classical mechanics by finding the laws of motion for solids and of gravitation between masses. This theory was so successful for the deterministic description of motions, in particular for the planets in our solar system, that Newton was led to the assumption that also light has corpuscular character. On the basis of light particles, which propagate along a straight line in a light beam, he could consistently explain a number of optical phenomena including the reflection and diffraction of light. The diffraction and interference experiments of Christian Huygens living at Newtons time and a little bit later, at the beginning of the 19th century, of Thomas Young and Augustin Fresnel, however, paved the way for the wave theory of light, at that time still waves in a not understood ether.
The triumph of wave theory could not be stopped anymore when the prominent Scottish physicist James Clark Maxwell successfully described the nature of light by a wave-like propagation of electrical and magnetic fields. He, thus, unified the two classical branches of optics and electricity in one and the same theory. By the detection of radio waves at around 1887, Heinrich Hertz finally established the familiar theoretical system of electrodynamics and electromagnetic waves.
Simultaneously, during the 19th century, an atomistic and molecular view of matter emerged and became more and more important, and this against various philosophical objections. Milestones in the development of an atomistic picture of matter were certainly the statistical kinetic gas theory of Ludwig Boltzmann around the end of the 19th century and the explanation of the Brownian motion in terms of collisions between liquid molecules and pollen particles suspended in the liquid by Einstein in 1905.
At the beginning of the 20th century, then, experimental results accumulated which contributed essentially to the emergence of a new physics, quantum physics. Among these there must be mentioned the detection of cathode rays in vacuum tubes, of X-rays and of radio activity. In particular, the Rutherford model of the atom must be emphasized, which was suggested by Ernest Rutherford in order to explain his scattering experiments of
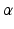
-particles on metal foils. Rutherfords atom is already imagined to consist of a massive small nucleus containing almost the entire atomic mass and an extended electronic cloud which determines the spatial extension of the atom.
This breakthrough in the understanding of the atom might be thought of as the beginning of the era of quantum physics. In a next step, the emission of sharp spectral lines of exited atoms being in contradiction to the successful theory of electrodynamics by Maxwell was explained. In 1913 Bohr interpreted, or better made plausible, the emitted line spectrum of hydrogen atoms on the basis of heuristic postulates about stable electron orbits around the positive nucleus, the proton.
A little bit earlier, already Max Planck had broken new ground into the direction of quantum physics. Around the end of the 19th century there was the puzzle of black body radiation. A so-called black body emits a continuous spectrum of electromagnetic radiation whose shape strongly depends on the temperature of the emitter. By means of classical electromagnetic theory, the spectrum for the shortest wavelengths always was calculated to diverge into infinity, the so-called ultraviolet (UV) catastrophe. Planck, who was a quite conservative physicist, made the revolutionary assumption that a black body interacts with the electromagnetic field by exchange of energy only in small quanta rather than in a continuous way. The UV catastrophe could thus be removed and the experimental black body emission theoretically be described correctly. In a kind of desperation, he must have drawn this conclusion which was in strict contradiction to Maxwells electromagnetic field theory of continuous electric and magnetic fields. The assumption, indeed, led back to the rejected corpuscular theory of light by Newton. Planck created the term quantum which gave the whole field its name. In his theoretical assumption, the quanta carry an energy E which is proportional to the light frequency
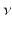
. The constant
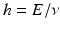
has been named Plancks constant in honor of its inventor. A number of illuminating detections followed (Chap. ) shall be mentioned.
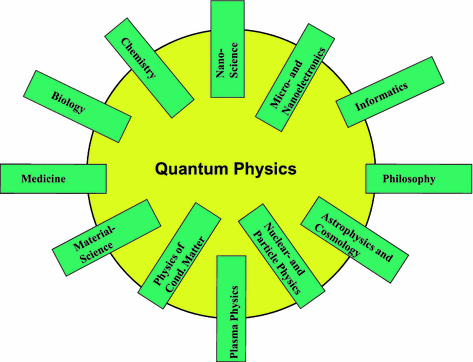
Fig. 1.1
Qualitative representation of the overlap between important science branches and the field of quantum physics. The amount of overlap with the quantum circle indicates how far quantum physical methods, theoretical and experimental ones, are used in the particular science disciplines
1.2 Importance for Science and Technology
While quantum theory was originally intended to explain the world of atoms, molecules and elementary particles, in particular the electron, it became clear meanwhile, that the theory has universal importance for the understanding of the whole surrounding world, up to cosmological questions. This is by no means astonishing since our world consists of atoms, elementary particles and energy fields which closely interact with matter. Thus, the stability of matter can only be understood on the basis of quantum theory (Sect. ).
The fundamental principles of quantum theory as particle-wave duality, the uncertainty principle and the random behavior on the atomic level, therefore, have to be taken into account in almost every natural or engineering science. This is true, although, because of historical or practical reasons, models of classical physics, mechanics or chemistry are used in many of these sciences. This is shown in a somewhat qualitative way in Fig. ) only indicates qualitatively to what extent one uses typically quantum physical methods and considerations in this field. Partially, this is dependent on the degree of atomistic thinking in a particular science field.










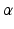 -particles on metal foils. Rutherfords atom is already imagined to consist of a massive small nucleus containing almost the entire atomic mass and an extended electronic cloud which determines the spatial extension of the atom.
-particles on metal foils. Rutherfords atom is already imagined to consist of a massive small nucleus containing almost the entire atomic mass and an extended electronic cloud which determines the spatial extension of the atom.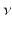 . The constant
. The constant 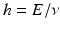 has been named Plancks constant in honor of its inventor. A number of illuminating detections followed (Chap. ) shall be mentioned.
has been named Plancks constant in honor of its inventor. A number of illuminating detections followed (Chap. ) shall be mentioned.