1.1.1 Generation Via ,-Unsaturated Aldehydes for Construction of Heterocycles
Carbenes possessing a bivalent carbon atom with two nonbonding electrons are considered as highly reactive intermediates, and their isolation has been a challenge for a long time. The synthesis of stable imidazolium carbenes was realized by Arduengo and co-workers in 1991 [].
The first example of a N-heterocyclic carbene-catalyzed reaction can date back to 1943, Ukai and co-workers initially reported the thiazolium salts-catalyzed benzoin reaction of aldehydes [].
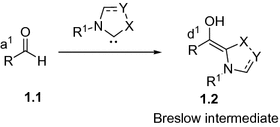
Fig. 1.1
NHC-catalyzed generation of Breslow intermediate
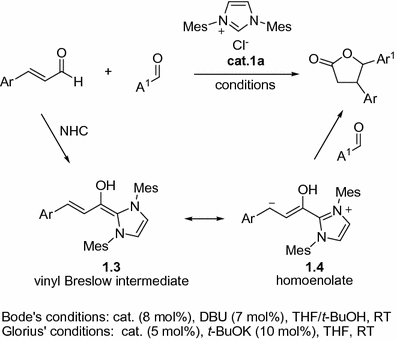
In 2004, Bode et al. [). After that, NHCs were found to be efficient catalysts for various annulations involving ,-unsaturated aldehydes. The conjugate umpolung of ,-unsaturated aldehydes via homoenolate equivalents opened a new access for the annulation reactions catalyzed by NHC.
Fig. 1.2
NHC-catalyzed [3+2] annulations of enals with aldehydes
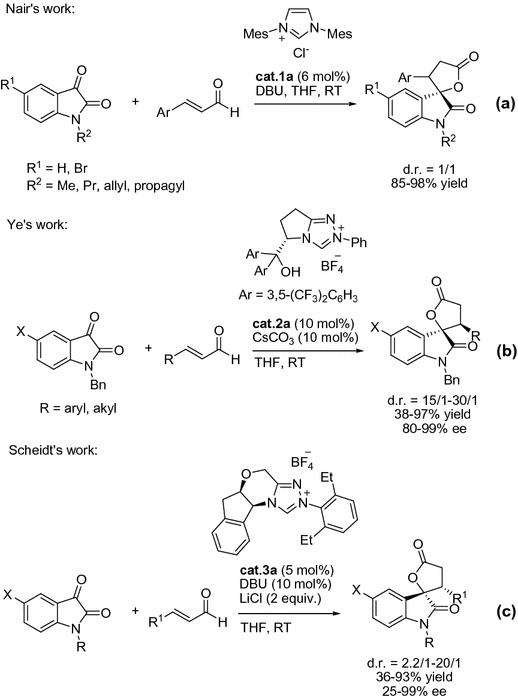
In 2006, Nair et al. [].
Fig. 1.3
NHC-catalyzed [3+2] annulations of enals with isatins
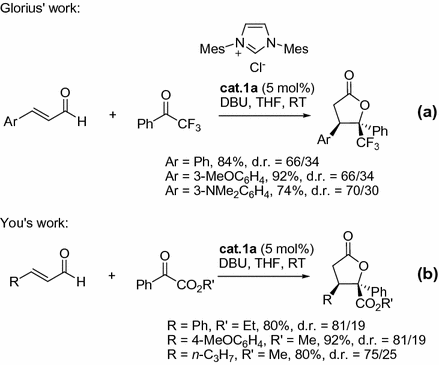
In further investigations, this concept was extended to generate -butyrolactones bearing quaternary stereocentres by Glorius and co-workers [).
Fig. 1.4
NHC-catalyzed [3+2] annulations of enals with ketones
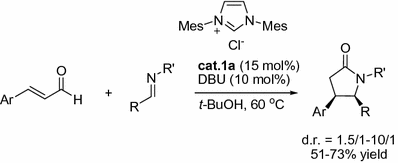
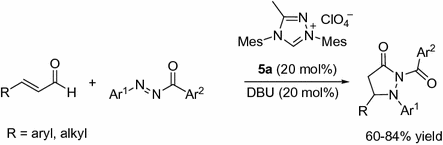
Employing a similar strategy, Bode and co-workers developed a variation on the reaction system via condensation of imines and homoenolates [).
Fig. 1.5
NHC-catalyzed [3+2] annulations of enals with imines
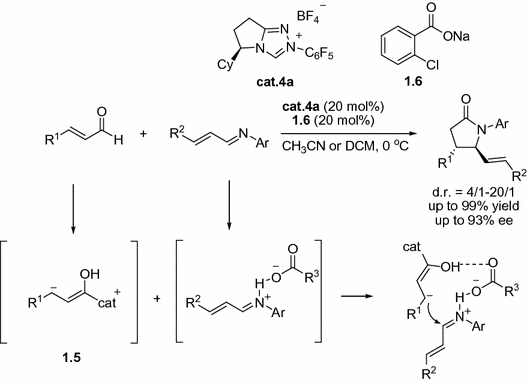
The simultaneous use of acid with an NHC is a real challenge. A major breakthrough was made in 2011, Rovis and co-workers introduced the Bronsted acids/NHC co-catalysis, which opens new opportunities for NHC catalysis. They found that the use of the NHC precursor triazolium salt cat.4a with catalytic amount of carboxylate 1.6 could activate the imines, improving the enantioselectivity and yield of the reaction (Fig. ].
Fig. 1.6
Bronsted acids/NHC co-catalysis for the synthesis of trans --lactams
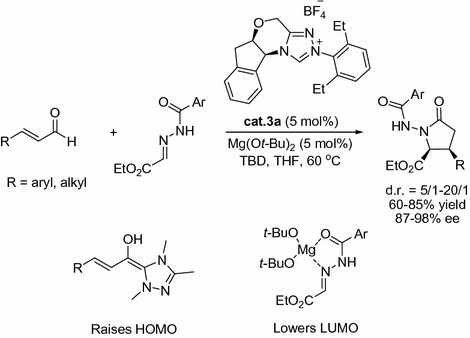
Its well known that NHCs are good ligands for metals. Its interesting that Scheidt and co-workers reported that the NHC precursor triazolium salt cat.3a in combination with catalytic amount of Mg(O t -Bu)2 could afford cis --lactams in good to excellent yields and ee via homoenolates (Fig. ].
Fig. 1.7
Lewis acids/NHC co-catalysis for the synthesis of cis --lactams
Besides aldehydes, ketones, imines, NHCs are also powerful catalysts for [3+n] annulations of enals with other electrophiles via homoenolate. For example, Scheidt and co-workers developed NHC-catalyzed [3+2] annulations of enals and azodicarboxylates, giving the corresponding pyrazolidinone products in good yields (Fig. ].
Fig. 1.8
NHC-catalyzed [3+2] annulations of enals with azodicarboxylates
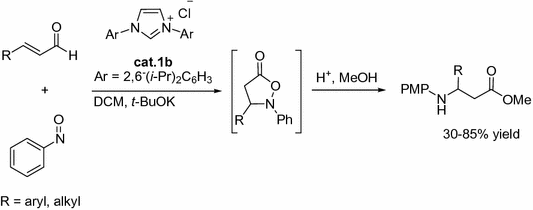
In the same year, The NHC cat.1b was demonstrated to be an efficient catalyst for the reaction of enals and nitroso compounds by Ying and co-workers (Fig. ].
Fig. 1.9
NHC-catalyzed [3+2] annulations of enals with nitroso compounds
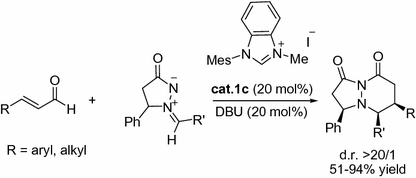
The highly selective [3+3] annulation reaction of enals with azomethine imines catalyzed by N-heterocyclic carbenes was also demonstrated by Scheidt and co-workers. The corresponding bicyclic heterocycles were constructed in good yields with excellent diastereoselectivity under the catalysis of cat.1c (Fig. ].
Fig. 1.10
NHC-catalyzed [3+3] annulations of enals with azomethine imines
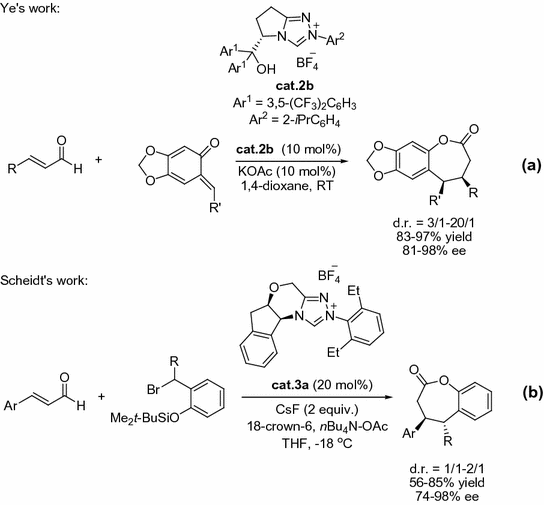
Subsequent to these previous reports, Ye et al. [).
Fig. 1.11
NHC-catalyzed [3+4] annulations of enals
1.1.2 Generation Via ,-Unsaturated Aldehydes for Construction of All Carbon Cycles
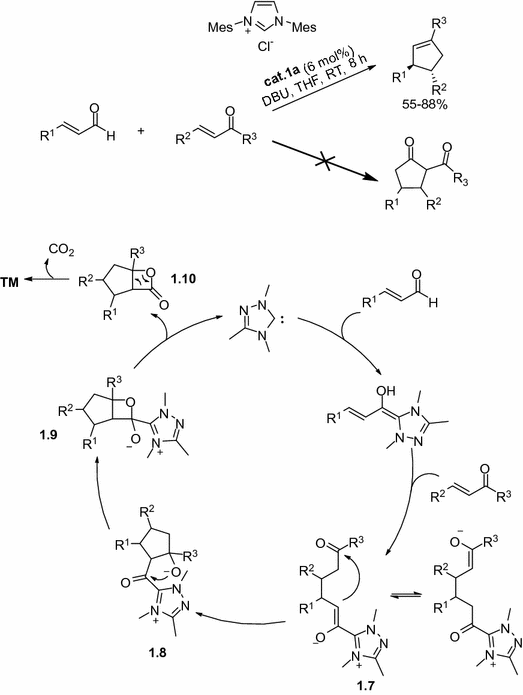
Furthermore, the homoenolate equivalent could be extended to construct all carbon cycles via homoenolate/enolate domino reactions. In 2006, Nair and co-workers pioneered the NHC-catalyzed [3+2] annulation of enals and chalcones, diverse cyclopentenes were afforded via this method [, the Michael addition of homoenolate to chalcone gave adduct 1.7 , followed by intramolecular aldol reaction with the activated carbonyl group. The delivered adduct 1.9 led to a -lactonization and regenerated the catalyst. The decarboxylation of unstable -lactone 1.10 gave the final cyclopentene via releasing an equivalent of CO2.
Fig. 1.12
The domino homoenolate/enolate annulations
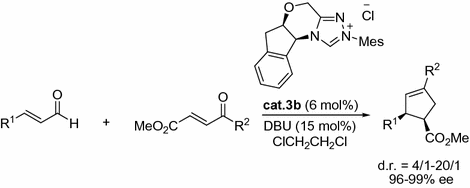
The enantioselective annulation of 4-oxoenones with enals was successfully developed by Bode and co-workers. In the presence of NHC cat.3b , the cis -cyclopentenes were afforded in good yields with high ee and good dr (Fig. ].
Fig. 1.13
Enantioselective synthesis of cis -cyclopentenes