Copyright 2016 Andy Brunning. All rights reserved. Any unauthorized duplication in whole or in part or dissemination of this edition by any means (including but not limited to photocopying, electronic devices, digital versions and the Internet) will be prosecuted to the fullest extent of the law.
Published in the US by
ULYSSES PRESS
P.O. Box 3440
Berkeley, CA 94703
www.ulyssespress.com
First published as Why Does Asparagus Make Your Wee Smell? in Great Britain in 2015 by Orion, an imprint of the Orion Publishing Group Ltd., an Hachette UK Company
ISBN: 978-1-61243-591-6
US editor: Alice Riegert
Design concept by Grade Design
Cover design by what!design @ whatweb.com
Cover photos from Shutterstock.com: asparagus ksulee; beer Serhiy Shullye; bacon Joe Belanger; GMEVIPHOTO; red onion Amero; red hot pepper Timmary; abstract background Tusiy
Interior photo credits: see
Distributed by Publishers Group West
IMPORTANT NOTE TO READERS: This book is independently authored and published and no sponsorship or endorsement of this book by, and no affiliation with, any trademarked brands or other products mentioned or pictured within is claimed or suggested. All trademarks that appear in ingredient lists, photographs and elsewhere in this book belong to their respective owners and are used here for informational purposes only. The authors and publishers encourage readers to patronize the quality brands mentioned and pictured in this book.
To Anna, for her continued support and enthusiasm throughout the creation of this book.
Table of Contents
Guide
CONTENTS


Food plays a huge part in our everyday lives, but we hardly ever stop to think about the science behind it. We all know that chopping onions makes you cry, that eating garlic gives you bad breath and that mint makes your mouth feel cool, but most people dont have a clue how to describe the chemical reasons behind these strange effects. The aim of this book is to look at the quirky and sometimes downright weird properties that food and drink can exhibit, and explain in simple terms the chemistry that leads to them.
In our explanations, were going to be talking primarily about organic chemistry. This use of the word organic is different from the one that we usually associate with food in supermarkets; in chemistry, it refers to chemical compounds based on carbon. There are many millions of possible organic compounds, with a wide range of different properties. You, yourself, are made up of organic compounds, and so are the foods we eat. These compounds are responsible for the flavor and aroma of the foods and drinks we consume on a daily basis, and can also help explain some of the different effects that we see.
The hope is that the material in this book will be accessible even to those who only have a basic understanding of chemistry. There is a brief introduction on the next page to some of the chemistry, to help with interpretation of the structures and descriptions later in the book.
If you do have a background in chemistry, and want to read in further detail on some of the topics examined here, references to the studies consulted during the writing of this book are also given at the end.
Whatever your interests or curiosities, I hope that the chemistry in this book will turn the everyday into the extraordinary for you.

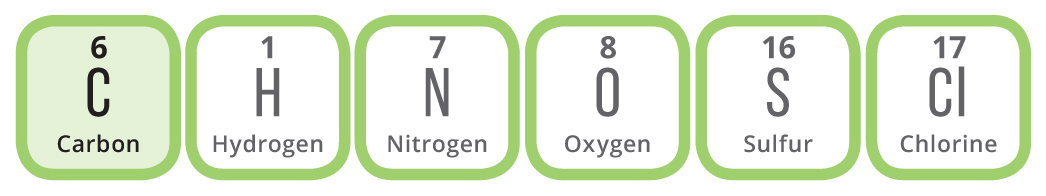
Organic chemistry is the study of carbon-based compounds. Organic compounds usually contain primarily carbon and hydrogen, but well also see some containing the other elements shown here.
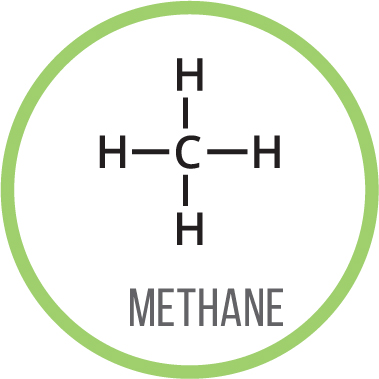
The chemical bonds in organic compounds are formed by the atoms sharing a pair of electrons with each other. Carbon can form four bonds to other atoms; oxygen usually forms two bonds, while hydrogen can only form one bond. Bonds are shown as lines; two lines between atoms indicates a double bond, or two shared pairs of electrons.
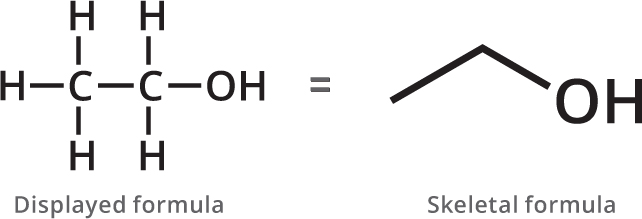
Organic compounds can be drawn showing all their bonds and all their atoms, as on the left. However, for bigger molecules, this can end up looking pretty messy so we commonly use the skeletal formula to represent molecules.
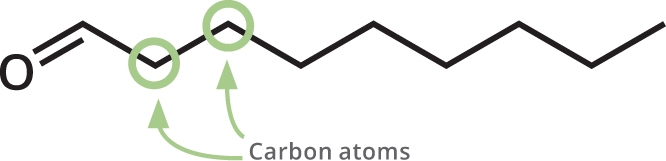
The skeletal formulas show each carbon atom as a bend in the chain of the molecule. Hydrogen atoms attached to carbons arent shown, to make the structure easier to interpret. Atoms other than carbon and hydrogen are shown.
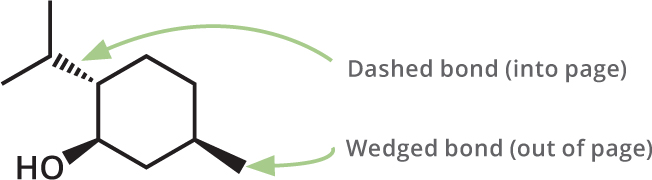
While chemical structures are shown in two dimensions on the page, obviously they are three dimensional in real life. In some cases, it is useful to indicate this in structures, so for this purpose we sometimes use dashed bonds, to show a bond going away from you and into the page, and wedged bonds, to show a bond coming toward you and off of the page.

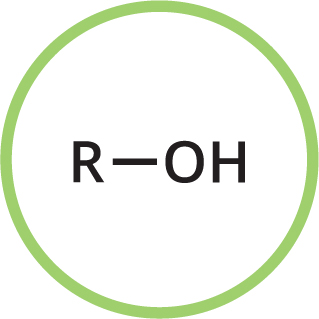
Functional groups are groups of atoms in organic compounds that are responsible for the characteristic reactions and properties of those compounds. In many compounds in this book, you will see several of these groups in one molecule. The functional groups present in a molecule are usually reflected in its name, as indicated here.
You may also see R used in some moleculesthis represents a further part of the molecule that may be variable. If you see X used, it indicates a halogen atom (the halogens are the elements fluorine, chlorine, bromine and iodine). Some typical functional groups are shown here.
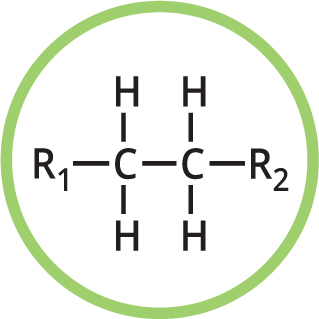
ALKANE
Naming: -ane e.g., ethane
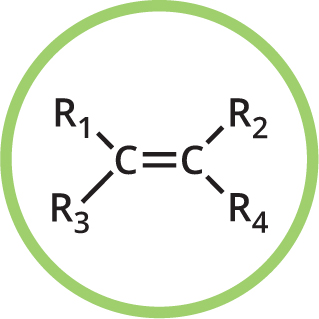
ALKENE
Naming: -ene e.g., ethene