Springer International Publishing AG 2018
Mark K. Friedberg and Andrew N. Redington (eds.) Right Ventricular Physiology, Adaptation and Failure in Congenital and Acquired Heart Disease
1. Embryological Origins: How Does the Right Ventricle Form
Paul Delgado-Olgun 1
(1)
Translational Medicine, Peter Gilgan Center for Research and Learning, The Hospital for Sick Children, 686 Bay St Room 10-9715, Toronto, ON, Canada, M5G 0A4
Abstract
The heart originates from a group of cardiac progenitor cells that form the cardiac tube, which develops into a complex four-chambered beating organ. Several tissues signal to stimulate cardiac progenitors to acquire cell fate and differentiate. The timing of differentiation of cardiac progenitors defines the first and second heart fields. The first heart field gives rise to the left ventricle. The second heart field, located anterior to the first heart field, is added to the cardiac tube to give rise mainly to the outflow tract and the right ventricle. Several epigenetic mechanisms including histone and DNA methylation stabilize the transcriptional programs controlling cardiac development.
Keywords
Cardiac development Second heart field Cardiac progenitors Right ventricle Cardiomyocyte differentiation Transcriptional regulation
Introduction
The heart develops from cardiac progenitor cells that originate during gastrulation and which move through the primitive streak and emerge as a bilateral cardiac field that fuses in the embryonic midline to form the cardiac crescent and the heart tube later on. The cardiac tube, in which cardiac progenitors start differentiating into cardiomyocytes, serves as a scaffold for addition of cardiac progenitors through the arterial and venous poles. The cardiac tube undergoes complex morphogenetic movements including looping and septation that result in the division of the heart into chambers []. The exact nature of the molecular cues that induce segregation of these progenitors is not clear, however extensive research using mainly animal and cellular model systems has made significant progress in uncovering the transcriptional pathways that regulate differentiation of cardiac progenitors and cardiogenesis.
This chapter provides an overview of the process of cardiac development, starting with a concise explanation of the morphogenetic events that transform the cardiac crescent into the four-chambered heart. Then, the events that led to the identification of the cardiac fields are discussed, emphasizing the discovery of the second heart field and its contribution to cardiogenesis. The transition from proliferating to differentiating cardiac progenitors and its relevance for development of the second heart field and right ventricle is discussed. Finally, recent findings on the molecular mechanisms controlling differentiation and maturation of cardiac myocytes are summarized.
Overview of Cardiac Development
Early Cardiac Development
The heart is the first organ to function during embryogenesis. The formation of the heart is a complex process that starts very early during development. The earliest cardiac progenitors arise during gastrulation [].
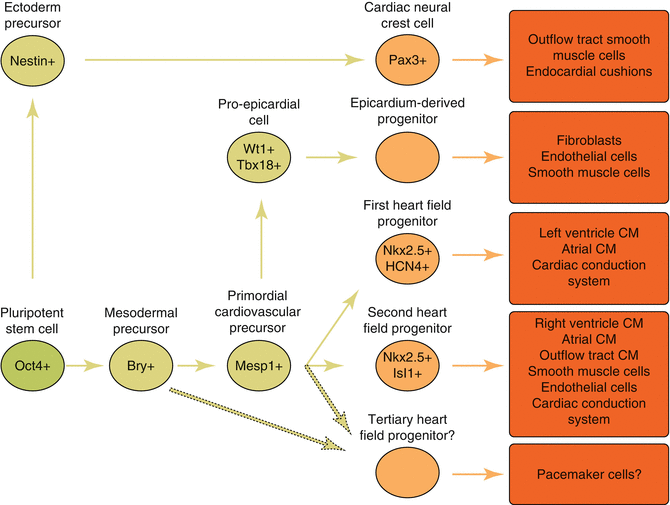
Fig. 1.1
Cardiac cell lineage progression . Intermediate stages generated from pluripotent stem cells towards differentiated cardiac lineages. Markers expressed in the intermediate progenitors are indicated. CM cardiomyocytes. Progression towards tertiary heart progenitors is speculative and is indicated with dotted arrows . Modified from []
The cardiac progenitors emerge through the primitive streak and they take position cranially to the forming neural tube and surrounding the neural plate at approximately 18 days of human development. The cardiac progenitors aggregate to form two bilateral primitive heart fields, which fuse in the midline to form a horse shoe-shaped tube from splanchnic mesoderm known as the cardiac crescent. The cardiac crescent harbors a population of cardiac progenitors known as the first heart field, which contributes to the formation of the left ventricle and portions of the atria. As a result of ventral folding of the embryo in a cranial to caudal direction, the limbs of the cardiac crescent coalesce and fuse in the midline to form the linear heart tube (Fig. ].
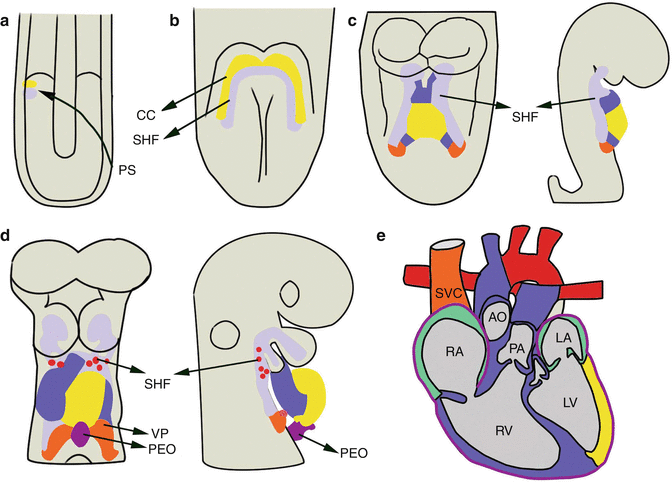
Fig. 1.2
Cardiac development. ( a ) Cardiac progenitors migrate anteriorly from the primitive streak (PS) to form two bilateral cardiac fields that ( b ) fuse in the midline to form the cardiac crescent ( yellow ) with the second heart field ( purple ) located medially. ( c , d ) Front ( left ) and lateral ( right ) views of the ( c ) linear, and ( d ) looping heart tube. Progenitors of the second heart field and the neural crest ( red ) migrate into the looping heart. ( e ) Fully developed heart. Green colored atria represent contribution of first and second heart field progenitors. The proepicardial organ gives rise to the epicardium. AO aorta, CC cardiac crescent, LA left atrium, LV left ventricle, PEO proepicardial organ, PA pulmonary artery, SHF second heart field, RA right atrium, RV right ventricle, SVC superior vena cava, VP venous pole. ( a d ) modified from []
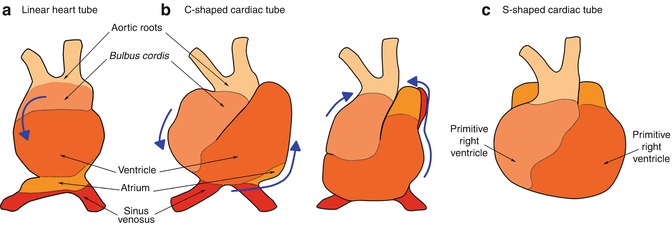
Fig. 1.3
Cardiac looping . The linear heart tube ( a ) twists and rotates rightwards resulting in the formation of the C-shaped tube ( b ). Elongation at the arterial and venous poles force arrangement into the S-shape tube ( c ), in which the outflow and inflow tracts come closer together cranially. Displacement of the bulbus cordis caudally, ventrally and rightwards, leftwards displacement of the primitive ventricle, and dorsal and cranial displacement of the primitive atrium, results in the proper spatial arrangement of the future cardiac chambers. Modified from []
Cardiac Looping
Looping of the heart tube is the first visual evidence of embryonic asymmetry. Progenitors of the second heart field continue to be added during the process of looping, in which torsion forces cause the elongating heart tube to simultaneously twist and rotate rightwards, resulting in the formation of a C-shaped cardiac tube at embryonic day 23 (Fig. ].
After looping is complete, cells from the proepicardial organ (Fig. ].
Cardiac Septation
The process of cardiac septation has been extensively reviewed [].
Ventricular Septation
Septation of the common ventricle occurs during the fifth week of development, and is completed by the ninth week. The muscular projection that will separate the ventricle starts forming during the looping process, when the walls of the future right and left ventricles grow concomitantly and coalesce resulting in the formation of the primitive interventricular septum, or interventricular ridge, at the base of the common ventricle [].
The molecular processes patterning the ventricles are poorly understood. Comparative studies of the expression of the developmental regulator Tbx5 , which is a key transcription factor regulating cardiac differentiation, have provided interesting insight. While Tbx5 is homogenously expressed in the early developing single ventricle of turtle and lizard, it is restricted to precursors of the left ventricle in chicken and mouse. In later stages of development, Tbx5 is preferentially expressed in a left to right gradient in the turtle ventricle, which by then develops an interventricular primary septum-like structure. Consistent with a key function for Tbx5 in cardiac septation, genetically modified mice that mimic the reptilian Tbx5 expression pattern develop a single ventricle. Thus, expression of Tbx5 may constitute a patterning cue for ventricular septation [].