1.1 Introduction
Human beings have always been obsessed with controlling extreme temperatures and apparently all for one purpose: eating. At one end of this thermic spectrum, mans discovery of fire marked a crucial turning point in human evolution. For some authors, cooking made man [], published in 1550, uses the word refrigerant for the first time, mentioning some of its uses.
However, if cold was to be used to cure diseases, lower temperatures were needed. This marked the beginning of the cold race: the quest for the perfect refrigerant had begun.
1.2 When Ice Met Salt (20 C): Arnott, the First Crusader of Cryosurgery
The first record of a formal medical application of controlled freezing to destroy tissues is found by James M. Arnott (Scotland, 17971883), an English physician who used a technique that allowed him to reduce tissue temperature to the proximity of 20C. Between 1819 and 1879, he published on the use of cold as a medical treatment [). He wrote that very low temperatures would stop any inflammation process as long as it was kept in close contact with the cold source for a long enough period of time. Although he started using cold for palliation of tumors, he ended up using it for many skin, breast, and uterine cancers; he was afraid of the pernicious side effects of chemicals used as early anesthetics and preferred the anesthetic power of cold for reducing pain.
Fig. 1.1
General view of the Great Exhibition of London of 1851, where Arnott received the prize medal for original equipment that allowed reducing tissue temperature to 20C ( Source : http://upload.wikimedia.org/wikipedia/commons/e/eb/Crystal_Palace_interior.jpg . With permission of collections.vam.ac.uk. Artist: McNeven J. Lithographer: William Simpson. The transept from the Grand Entrance, Souvenir of the Great Exhibition. Publisher: Ackermann & Co: 1851)
1.3 End of the Nineteenth Century: The Race for Liquefied Gases Begins
Mixtures of crushed ice with salts were, however, not capable of reducing tissue temperature enough to destroy tumor cells. Lower temperatures were needed, and they were finally attained in the last part of the nineteenth century thanks to the discovery of the necessary technology to liquefy gases. First it was oxygen and then nitrogen. Raoul-Pierre Pictet (Switzerland, 18461929) (Fig. ]. Pictet and Cailletet share credit of being the first men to liquefy an atmospheric gas.
Fig. 1.2
Raoul-Pierre Pictet (Geneva, Switzerland, 18461929) ( Source : Edgar Fahs Smith Collection, University of Pennsylvania Libraries. http://sceti.library.upenn.edu/sceti/smith/imagedetail.cfm?PictureID=1303&position=1&keywords=pictet&subcoll=scientist . In Public Domain)
Fig. 1.3
Louis-Paul Cailletet Laperouse (Chtillon-sur-Seine, Cte-dOr, France, 18321913) ( Source : Edgar Fahs Smith Collection, University of Pennsylvania Libraries. http://sceti.library.upenn.edu/sceti/smith/imagedetail.cfm?PictureID=1390&Position=2&keywords=cailletet&subcoll=scientist . In Public Domain)
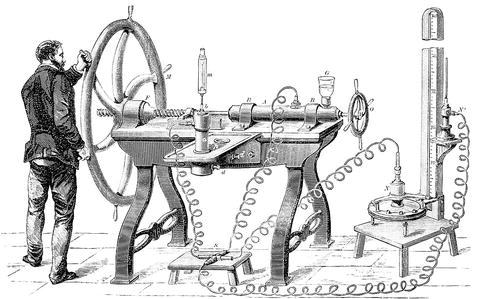
Fig. 1.4
Image of Cailletets gas liquefaction apparatus ( Source : http://upload.wikimedia.org/wikipedia/commons/1/1e/PSM_V12_D635_Cailletet_gas_liquefaction_apparatus.jpg . The Popular Science Monthly , No 12, Nov 1877 to April 1878. New York: D. Appleton and Company, 1879. https://archive.org/details/popularsciencemo12newy ; In Public Domain)
Carl von Linde (Germany, 18421934) (Fig. ]. By 1871, he built his first ammonia compression machine, and by 1905 he obtained pure oxygen and nitrogen. He was the first to create a continuous process for liquid gas production. Linde became an expert in refrigeration and gas separation technologies and was the founder (1879) of what is today a leading multinational gases corporation.
Fig. 1.5
Carl von Linde (Upper Franconia, Germany, 18421934) (From Edgar Fahs Smith Collection, University of Pennsylvania Libraries. http://sceti.library.upenn.edu/sceti/smith/imagedetail.cfm?PictureID=271&position=1&keywords=linde&subcoll=scientist . In Public Domain)
In 1891 the physical chemist James Dewar (Scotland, 18421923) (Fig. ] built a machine capable of making liquid oxygen in large quantities. Dewar worked throughout his life on the behavior of gases at very low temperatures. He made liquid forms of many gases and designed many devices to produce them or to store them. In 1898, Dewar condensed liquid hydrogen with the help of one of the devices he had invented, the Dewar flask. He needed a place to store the liquefied gases he produced. In 1892, he developed the idea of a flask with two silvered walls separated by an evacuated air chamber, thus insulating the inside from the outside of the flask. He knew heat transfer occurred by three physical phenomena: convection, conduction, and radiation. Vacuum eliminated convection, since there are no moving molecules in the gas to transfer heat, and also greatly reduced conduction, as there would be far fewer adjacent gas molecules. Dewars invention allowed for the first time the use of liquid gases outside the laboratory, thus making liquefied gases accessible to physicians. In his honor or perhaps unknowingly, the insulating storage vessel commonly used to keep liquid nitrogen is still called Dewar flask.
Fig. 1.6
James Dewar (Kincardine-on-Forth, Scotland, 18421923) (From Edgar Fahs Smith Collection, University of Pennsylvania Libraries. http://sceti.library.upenn.edu/sceti/smith/scientist.cfm?PictureID=611&ScientistID=477 . In Public Domain)
Zygmunt Florenty Wrblewski (Russian Empire, today Belarus, 18451888) (Fig. ] were the first scientists reporting liquefaction of oxygen, nitrogen, and carbon dioxide in a stable condition (1883). At the Jagiellonian University in Krakow, where the two scientists met as professors, Olszewski had started his experiments compressing and condensing carbon dioxide. In 1884, Olszewski was the first to liquefy hydrogen in a dynamic state at 225 C. Wrblewski died of severe burns (1888) during an experiment to study the properties of hydrogen. Olszewski liquefied argon in 1895, but he failed at liquefying helium. In 1886, just a few days after learning about Wilhelm Rntgens work on X-rays, Olszewski replicated the experiment.