De Gruyter Textbook
ISBN 9783110656145
e-ISBN (PDF) 9783110656268
e-ISBN (EPUB) 9783110656411
Bibliographic information published by the Deutsche Nationalbibliothek
The Deutsche Nationalbibliothek lists this publication in the Deutsche Nationalbibliografie; detailed bibliographic data are available on the Internet at http://dnb.dnb.de.
2022 Walter de Gruyter GmbH, Berlin/Boston
Dedicated to Parents, Husband and Son
About the author

Born in April 20, 1971, at Bhilai in Madhya Pradesh, Dr. Vandana Sakhre graduated in chemical engineering from the National Institute of Technology (NIT), Raipur, in 1994. She obtained her postgraduate degree in environmental science and engineering from the Indian Institute of Technology (IIT), Dhanbad, in 2002. She obtained her doctorate degree in chemical engineering in December 2016 from Rajiv Gandhi Technical University (RGPV), Bhopal (Madhya Pradesh). Dr. Vandana Sakhre has over 23 years of experience of teaching, research, and industry, which includes 18 years as assistant professor, 2.5 years of industrial experience, and 3 years as research fellow of CSIR Labs. During teaching, she has developed various labs, including heat transfer, mass transfer and chemical reaction engineering and process dynamics and control labs. The advanced separation and research lab for MTech program was also developed.
Currently, Dr. Vandana is working with Manipal Academy of Higher Education, Dubai campus, as assistant professor-selection grade since 2017. She is handling Chemical Engineering Department as program coordinator and the coordinator of students project and research work. She is presently working on BASF project as an external funded project, and she did few internally funded research projects. She is dedicated to teaching, learning, and research.
Dr. Vandana has worked on artificial intelligence techniques for synthesis and control of chemical separation processes, especially reactive distillation (RD). She is also engaged in research on sustainability and environment management. In this book, she is giving overview and case studies on RD.
Chapter 1 Introduction
1.1 Distillation process
In the present scenario, distillation plays an important role in process industry. It is a popular separation process in chemical, petrochemical, and other industries. Its applications are in separation of crude oil into its fractions, purification of solvents, distillation of air, water, and fermented solutions, and many more. Distillation is a separation process which is based on differences in boiling points or principle of relative volatilities of the components present in the liquid mixture. Heat is given to the system so that components get separated based on their boiling points to the desired purity; hence, it is an energy-intensive separation process. The greater the difference in volatility of the component, it is easier to separate the component from their mixture. There are two types of distillation process: binary distillation and multicomponent distillation process. In binary distillation, separation of mixtures of two components takes place, while in case of multicomponent distillation, separation of more than two components takes place.
In chemical process industries, the conventional sequence of manufacturing consists of reaction followed by separation/purification. The combination of reaction and separation into one single step/unit is known as reactive distillation (RD). RD is a process in which a reaction is combined with separation of product components using distillation in a single column. Two types of RD processes are considered for the present research work, that is, continuous RD process and reactive divided wall process [].
1.1.1 Continuous reactive distillation process
The combination of reaction and distillation performed in a single vessel is known as RD. Unlike the conventional reaction and separation process, RD is a combination of both in a single unit. Manufacturing of various chemicals like esters, ethers, cumene, and petroleum processing unit, required a reactor followed by a separator such as a distillation unit to separate the required product from other constituents based on relative volatility. There are various constraints on this type of processing like more space is required for the installation of the unit, higher cost, more energy input requirement, and reduced selectivity. Specifically, the conversion limits for reversible reactions are difficult to overcome toward highest purity of products because once the equilibrium is achieved in a system, no more reactants will be converted into products. In view of all these constraints, RD emerged as a novel technique of process intensification in which reaction and separation of products take place simultaneously in a single column.
The RD column is divided into three sections: the upper rectifying section, middle reaction section, and bottom stripping section. The reaction takes place in the reaction section while simultaneous separation of products formed takes place in rectifying and stripping sections. The continuous removal of products from the reaction mixture makes it feasible for equilibrium-limited reactions. The simultaneous removal of products also increases the rate of reaction and prevents any undesirable side reaction between reactants and products. The RD is also preventing the formation of azeotrope. The schematic diagram of RD column is shown in .
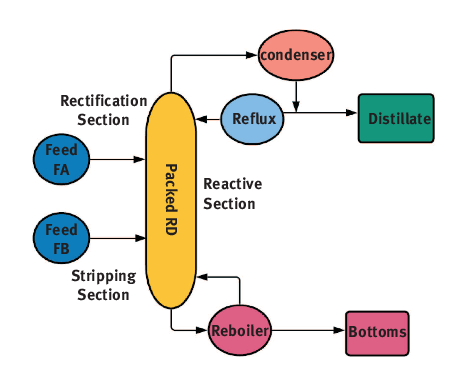
Figure 1.1: Schematic diagram of reactive distillation.
The most important benefit of RD technology is a reduction in capital investment because two process steps can be carried out on the single device. Such integration leads to lower costs in pumps, piping, and instrumentation. For exothermic reaction, the reaction heat can be used for vaporization of liquid. This leads to saving of energy costs by the reduction of reboiler heat duties [].
1.1.2 Reactive divided wall (RDW) distillation process
If a mixture of A, B, and C (in order of increasing boiling point) were to be separated, this could be done in one of three ways: direct, indirect, or transition split. In a direct split, component A will be the desired distillate, while a second column is required to separate B and C. In an indirect split, component C is obtained from the bottom, while A and B in the distillate require a further separation. In a transition split, A and B are from the distillate, while B and C are from the bottom. Then two more columns are needed, one for separating each of the original product streams. If these secondary columns are joined and the middle product B is taken as a side stream, and other side draws were sent back to the first column as recycles, this becomes what is known as a Petlyuk arrangement. When both columns of the Petlyuk arrangement are placed in the same shell, it becomes a dividing wall column. Combining the columns in one shell allows the removal of the condenser and reboiler from the pre-fractionators, with a consequent reduction in energy needs.
Reactive divided wall distillation column is a combination of RD and divided wall distillation column. In one part of the divided wall column, reaction takes place and the same heat of the reaction and the process is used to further separate the products in the other part of the column, thus increasing the productivity and reducing the energy requirements. Other design principles and the processes are similar as compared to RD and divided wall distillation column. Dividing wall columns have gained increasing application due to their lower energy consumption and lower investment costs compared with conventional distillation column sequences. However, because of the increased design degrees of freedom, optimal process design becomes exceedingly more difficult and remains an open issue. Also, the integrated nature of the column makes the process highly interacting, and a process control system must be designed with added care [.













