FOSSIL FUELS
ENERGY: PAST, PRESENT, AND FUTURE
FOSSIL FUELS
EDITED BY ROBERT CURLEY, MANAGER, SCIENCE AND TECHNOLOGY

Published in 2012 by Britannica Educational Publishing
(a trademark of Encyclopdia Britannica, Inc.)
in association with Rosen Educational Services, LLC
29 East 21st Street, New York, NY 10010.
Copyright 2012 Encyclopdia Britannica, Inc. Britannica, Encyclopdia Britannica, and the Thistle logo are registered trademarks of Encyclopdia Britannica, Inc. All rights reserved.
Rosen Educational Services materials copyright 2012 Rosen Educational Services,
LLC. All rights reserved.
Distributed exclusively by Rosen Educational Services.
For a listing of additional Britannica Educational Publishing titles, call toll free (800) 237-9932.
First Edition
Britannica Educational Publishing
Michael I. Levy: Executive Editor
J.E. Luebering: Senior Manager
Marilyn L. Barton: Senior Coordinator, Production Control
Steven Bosco: Director, Editorial Technologies
Lisa S. Braucher: Senior Producer and Data Editor
Yvette Charboneau: Senior Copy Editor
Kathy Nakamura: Manager, Media Acquisition
Robert Curley: Manager, Science and Technology
Rosen Educational Services
Jeanne Nagle: Senior Editor
Nelson S: Art Director
Cindy Reiman: Photography Manager
Matthew Cauli: Designer, Cover Design
Introduction by Laura Loria
Library of Congress Cataloging-in-Publication Data
Fossil fuels/edited by Robert Curley.
p. cm. (Energy: past, present, and future)
In association with Britannica Educational Publishing, Rosen Educational Services.
Includes bibliographical references and index.
ISBN 978-1-61530-540-7 (eBook)
1. Fossil fuels. I. Curley, Robert. II. Title. III. Series.
TP318.F67 2012
333.82dc22
2010045687
Cover (front top, back) Derricks drilling for oil; (front bottom) A consumer pumping gas. Shutterstock.com
Cover (front bottom) A consumer pumping gas. Shutterstock.com
On : Burning lumps of coal. Shutterstock.com
Pp. www.istockphoto.com/Teun van den Dries
CONTENTS









INTRODUCTION

F ossil fuels are of staggering significance throughout the world. Petroleum, natural gas, and coal are primary sources of energy that drive modern technology, affecting the lives of hundreds of millions of people. The production and sale of these fuels represent a billion-dollar-a-year industry, which greatly influences the global economy. Possession or, conversely, lack of these resources can sway the domestic and foreign policies of nations. Important resources such as these deserve careful consideration and in-depth analysis, which is the aim of this book. Within these pages lies a thorough analysis of the history, origins, production, and uses of fossil fuels.
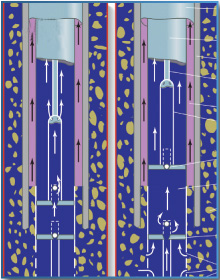
As their collective name indicates, fossil fuels are formed from the preserved remains of plants and animals, and are buried deep underground. Petroleum is composed of carbon and hydrogen that has been passed through an organic phase in single-cell plants or planktonic animals, such as blue-green algae or foraminifera. The preserved remains of such organisms become petroleum through a process known as diagenesis. The first stage of diagenesis involves the conversion of the remains to kerogen. With pressure, heat, and time, the kerogen is converted to petroleum at depths of 750 to 4,800 metres (2,500 to 16,000 feet), commonly referred to as the oil window. The mature oil moves through the pores and capillaries of porous sedimentary rocks such as shale, either seeping to the surface or accumulating in reservoir beds, or traps. Petroleum is classified by its predominant hydrocarbon. There are five grades of crude oil based on specific gravity, ranging from heavy to light, the latter being the most desirable. Light products can be recovered from heavy oil, but at a considerable cost.
Oil is refined, or separated into different fractions and sometimes chemically altered in preparation for use, through three basic processes. In the first, known as separation, hydrocarbons of specific properties are separated from the crude oil through distillation, with the oil vapours produced by the heat being condensed at the top of a tower unit. Next, molecular conversionfor example, through the process of catalytic crackingbreaks down the molecules, creating the desired product in greater volume. Finally, the purification stage removes contaminants through one of several treatment processes.
After crude oil is refined, a variety of products can be manufactured. Gasoline is the most common product; others include diesel fuel, fuel oils, and gases such as propane, or liquid petroleum gas (LPG). Gasoline must meet three requirements. It must have an even combustion pattern, to prevent engine knock, and allow the engine to start easily in cold weather. It also must meet changing environmental standards. Gasoline is graded with an octane rating, a number determined by taking the average score between two knock tests. The octane number, which for gasoline intended for automobiles ranges from 87 to 100, refers to the amount of octane that would be present in a fuel mixture whose performance matched the performance of the gasoline being tested in a knock engine. Gasoline contains a blend of up to 15 components with varying levels of volatility, to meet efficiency and environmental standards.
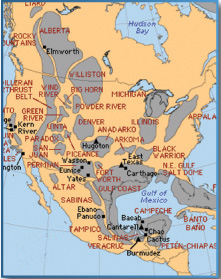
In the past, natural gas was erroneously considered merely a waste product of oil recovery processes. Both land plants and organic matter from the sea act as root material for the formation of natural gas. While petroleum is generated solely within the oil window, natural gas is much more pervasive; deposits are found above and below the oil window as well as within it. As with petroleum, natural gas migrates up from deep below Earths surface and accumulates in traps.
Next page























