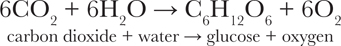The Chemistry
of Plants
T HIS IS THE equation that summarises what is unarguably the most important chemical reaction on Earth:
This reaction combines carbon dioxide and water to form energy-rich glucose, the simplest form of carbohydrate, upon which almost all life on Earth depends. Without carbohydrates, life as we know it would not be possible.
The reaction takes place in the green tissues of plants and is made possible only in the presence of the green pigment chlorophyll; it requires energy from the sun, which is then trapped within the carbohydrate molecule; the reaction is called photosynthesis.
It is the release of the energy trapped in the glucose molecule which provides the power required not just by the plant, but by almost all life to carry out all their daily functions: for plants to grow and reproduce; and for fungi and animals (including humans), who consume the glucose in plants. Even carnivores ultimately derive all their energy from plants via the herbivores lower in the food chain.
Carbon dioxide enters the plant a tree, in our case through the tiny pores, or stomata, on the surface of the leaves. Water is absorbed by the roots and is transported to the leaves by the xylem vessels of the stem and leaf veins.
Photosynthesis (creating glucose) takes place in the green tissues of the leaves. Glucose is then transported around the plant, in the phloem vessels of the veins of stems and roots, to where it is needed to provide energy for metabolic activity. It is used as building material for creating new tissues or stored as starch or oils for future energy needs.
Glucose provides building blocks, along with nitrogen and other elements obtained from the soil, to create all other major plant constituents, referred to as their primary metabolites: starches, proteins and lipids (oils). All three are found in all plants.
SPECIALISED METABOLITES
Plants also produce over 50,000 specialised metabolites (often referred to as secondary metabolites, a term that understates their remarkable properties and the unique roles they play). Although the specialised metabolites may not be fundamental to survival like the primary metabolites are, they serve a variety of functions to enhance the life of the plant, each one conferring an evolutionary advantage. Not all are produced by all plants most plants focus on just a few. Some are toxins used by plants to deter predation by herbivorous animals or pathogenic fungi; others might manifest themselves as pigments or volatile and scented oils designed, for example, to attract insects for pollination. Many of them have properties that benefit humans if consumed as part of the wider diet. Even those that are toxic can have medicinal benefits if taken in controlled doses.
It is the specialised metabolites that often provide the memorable, useful and sometimes dangerous characteristics of different plant families and individual species.
The subject of specialised metabolites is populated with a huge number of seemingly unrelated terms that you may have heard, particularly if you are interested in topics surrounding medicine, healthy food and aromatherapy. If you enjoy the marvels of organic chemistry, you will be disappointed not to find more detail here. But there is really not the space to explore the complexities of the subject so I have decided to keep the language of chemistry to a minimum. For those who have always hated chemistry please dont be put off by the occasional chemical term. You will generally find that the sentence makes sense if you ignore them.
To introduce the subject, I have highlighted four of the key terms alkaloids, glycosides, terpenes and polyphenols along with some relevant examples of each, most of which are associated with our trees and are referred to again in the species profiles later in the book. Note that the terms alkaloids, glycosides, terpenes and polyphenols are not all mutually exclusive from a chemistry perspective, which is why, for example, I refer to a terpene glycoside.
Alkaloids
There are over 21,000 alkaloids produced by plants, globally. Each alkaloid has a unique chemical structure. They usually taste bitter, are toxic to other organisms, and are used by the plant as grazing deterrents. Some famous plant alkaloids act on the central nervous system and thus have potential in traditional and modern medicine as analgesics, antihypertensives, tranquilisers, etc. Mankind has used alkaloids as stimulants (e.g. caffeine) or depressants (e.g. morphine) or for psychoactive experiences (e.g. cocaine) for thousands of years.
There is not a lot of evidence among our tree species of alkaloid occurrence. Globally, wind-pollinated families, including a third of our trees, tend not to produce alkaloids. But they do occur in conifers, notably the strong cardiotoxic, taxine alkaloids of Yew, Taxus baccata. They are located in the leaves and seeds and are therefore very effective anti-grazing chemicals. Globally, there seems to be a higher incidence of alkaloid occurrence in the more primitive families of plants that are listed early in the classification system. In this group we have only one member Box, Buxus sempervirens. One of its active ingredients is cyclobuxine, a steroidal alkaloid that occurs in the leaves and bark. This alkaloid has recently been studied for the treatment of HIV/AIDS and may have potential for Alzheimers and other conditions. Most alkaloids are lethally toxic in the wrong doses.
Polyphenols
There are over 7,000 polyphenols (aka phenolics). They include tannins and all the soluble so-called flavone pigments, some of which are antipathogenic, but most of which absorb damaging ultraviolet radiation and are used to attract and/or to deter. Of these, it is the anthocyanin pigments which give elderberries, and especially the dark cherries, their deep purple colour that attracts dispersal agents such as thrushes. Animals that enjoy consuming these fruits are seeking energy-rich sugars. They also benefit from associated nutrients including, in this case, the antioxidant qualities of the anthocyanins. Animals learn to associate the colour with the reward.
Tannins that give Crab Apples (particularly unripe apples) their bitterness are also phenolics. In this case the unpalatability prevents premature dispersal, before the seed is fully ready. The leaves, bark, wood and acorns of oak trees are famously rich in tannin, which acts as an effective inhibitor of fungal growth and hence decomposition. This has implications regarding carbon sequestration and the durability of timber. Oak timber is highly esteemed for its rot resistance.
Tannins have many diverse uses. Some of the polyphenol chemicals released from decomposing Sycamore leaves deter the germination and growth of young plants nearby. This competitive behaviour is known as allelopathy.
Glycosides
There are around 500 plant glycosides, which are a means for plants to store toxins in an inactive state until needed. The toxin is attached to a sugar molecule, which renders it inactive. When the leaf or another structure is damaged or infected, plant enzymes cause the sugar part to be broken off, thus activating the toxin. Cyanogenic glycosides protect the seeds of the Rose family, notably Bird Cherry, by releasing hydrogen cyanide if damaged. Normally, the seed is rejected or passes through the gut unharmed it is the fleshy surrounding fruit that is consumed. The iridoid glycoside of Dogwood (and many species in closely related families) deters grazers great and small, and the same chemical has been used pharmacologically as an effective anti-inflammatory. Despite the harmless, nutritious and fleshy outer seed coat (or aril) of Spindle, the seeds within are extremely toxic to birds and mammals, including man. They contain a cardiac glycoside (a terpene glycoside) and various other terpenes (see below). If consumed by humans, the concentration of these chemicals can cause severe liver and kidney damage and even death.