Air emissions include air pollution emissions and greenhouse gas (GHG) emissions. Air pollution emissions, which are commonly referred to as air pollutants, are species in the air that cause acute or chronic human health problems and negative environmental impacts. GHGs are not toxic unless at extremely high concentrations, but their higher-than-normal concentrations in the atmosphere may have resulted in global warming and climate change with possible catastrophic consequences on planet Earth.
Air pollution occurs when unwanted materials are added to the air, especially in abundance, resulting in effects on the environment and health. In principle, these unwanted materials may be anything in any phase, such as carbon dioxide (CO2), liquid droplets, and solid dust particles. A material is labeled as an air pollutant only when it is against the interest of human beings. For example, sulfur dioxide (SO2) is considered as an air pollutant due to its negative impact on the environment and human health, which will be elaborated on shortly.
In another example, if there is over 78 % of nitrogen in the air, it is not considered as an air pollutant, because it does not impose any noticeable negative effect on human beings or the environment. On the other hand, although CO2 has been a component of clean dry air (Table ) before human history, it was not labeled as an GHG emission until recently, when scientists observed a strong correlation between the increased level of atmospheric CO2 and global warming (or climate change), which could eventually eliminate the existence of human beings on this planet.
1.2.1 Air Pollution
Air emissions have natural or anthropogenic origins; however, this book focuses primarily on the latter, which include industrial activities and the burning of fossil fuels. These constituents of pollution have the potential to affect the majority of people in a region.
Air pollutants comprise primary and secondary air pollutants. Primary air pollutants are emitted directly from sources. They include, but are not limited to, particulate matter (PM), sulfur dioxide (SO2), nitric oxides (NO x ), hydrocarbon (HC), volatile organic compounds (VOCs), carbon monoxide (CO), and ammonia (NH3). Secondary air pollutants are produced by the chemical reactions of two or more primary pollutants or by reactions with normal atmospheric constituents. Examples of secondary air pollutants are ground level ozone, formaldehyde, smog, and acid mist.
Particulate matter is a mixture of solid particles and liquid droplets suspended in the air. In this book, particulate matter is interchangeable with aerosol, which is a suspension of solid or liquid particles in a gas. It is a two-phase system consisting of particles and the gas in which they are suspended. Particulate matter can be both primary pollutants and secondary pollutants that are sent directly into the atmosphere in the form of windblown dust and soil, sea salt spray, pollen, and spores. Other examples of PM are smoke, fumes, and haze.
For particulate matter where particle diameters are smaller than x micrometers, it is defined as PM x . Commonly used terms are PM10 and PM2.5. Sometimes, particulate matter and aerosol is exchangeable. Monodisperse aerosols, in which all particles have the same size, can be produced in laboratory for use as test aerosols. In practice, engineers deal with polydisperse aerosols (i.e. suspended particles are in a wide range of sizes), and statistical measures should be used to characterize particle sizes. Aerosol Technology by William Hinds (2006) is one of the reference books for this subject.
Air pollutants other than PM present primarily as gases. Volatile organic compounds (VOCs) are chemicals that contain carbon and/or hydrogen and evaporate easily. VOCs are the main air emissions from the oil and gas industry, as well as indoor consumer products and construction materials, such as new fabrics, wood, and paints. VOCs have been found to be a major contributing factor to ground-level ozone, a common air pollutant, and a proven public health hazard. Sulfur dioxide (SO2) and nitric oxides (NO x ) are two major gaseous air pollutants generated through combustion processes. Carbon monoxide (CO) and hydrocarbon (HC) are generated from incomplete combustion and are converted into CO2 through a complete combustion process.
Secondary air pollutants are those formed through complex physical and/or chemical reactions, e.g., coagulation and condensation or photochemical reactions, respectively, e.g.:
Atmospheric physics and chemistry are so complicated that they are still not well understood. Most gaseous air emissions such as NO x , SO2, HC, VOCs, and ammonia (NH3) are converted into PM. Readers are recommended to read Atmospheric Chemistry and Physics by Seinfeld and Pandis [] for in-depth knowledge on this subject.
Air pollution is an evolving subject and inevitable, as the demand for energy increases. Air pollution really flourished with the Industrial Revolution and continues to grow with the human appetite for comfort and speed.
At first, the study of air pollution focused on recurring episodes of high levels of air pollution in areas surrounding industrial facilities, such as coal burning power plants and chemical refineries. These pollution episodes were accompanied by acute human sickness and the exacerbation of chronic illness. After the mid-twentieth century, when industrialized nations economies recovered rapidly from World War II, many urban regions without heavy industrial facilities also began to experience high levels of photochemical smog and nitrogen oxides.
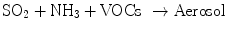





![Lex Fullarton - [T]axing Greenhouse Gases](/uploads/posts/book/299842/thumbs/lex-fullarton-t-axing-greenhouse-gases.jpg)