Buldakov Mikhail A. - Interaction-induced Electric Properties of van der Waals Complexes
Here you can read online Buldakov Mikhail A. - Interaction-induced Electric Properties of van der Waals Complexes full text of the book (entire story) in english for free. Download pdf and epub, get meaning, cover and reviews about this ebook. City: Cham, year: 2017, publisher: Imprint, Springer, Springer International Publishing, genre: Children. Description of the work, (preface) as well as reviews are available. Best literature library LitArk.com created for fans of good reading and offers a wide selection of genres:
Romance novel
Science fiction
Adventure
Detective
Science
History
Home and family
Prose
Art
Politics
Computer
Non-fiction
Religion
Business
Children
Humor
Choose a favorite category and find really read worthwhile books. Enjoy immersion in the world of imagination, feel the emotions of the characters or learn something new for yourself, make an fascinating discovery.
- Book:Interaction-induced Electric Properties of van der Waals Complexes
- Author:
- Publisher:Imprint, Springer, Springer International Publishing
- Genre:
- Year:2017
- City:Cham
- Rating:4 / 5
- Favourites:Add to favourites
- Your mark:
Interaction-induced Electric Properties of van der Waals Complexes: summary, description and annotation
We offer to read an annotation, description, summary or preface (depends on what the author of the book "Interaction-induced Electric Properties of van der Waals Complexes" wrote himself). If you haven't found the necessary information about the book — write in the comments, we will try to find it.
Buldakov Mikhail A.: author's other books
Who wrote Interaction-induced Electric Properties of van der Waals Complexes? Find out the surname, the name of the author of the book and a list of all author's works by series.










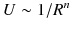 and the short-range interactions
and the short-range interactions 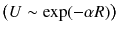 . The main long-range contributions to the energy and electric properties of interacting molecules are as follows: electrostatic (multipole-multipole), polarization (induction and dispersion), resonance, relativistic, magnetic and retardation. The short-range interactions include the direct electrostatic, exchange, repulsion, change transfer effects. Note that the long-range interactions can be well described in an analytical form using classical electrodynamics for some approximation of the perturbation theory. In turn, for short distances the methods of quantum chemistry are only effective. In part of analytical theory for large R this book will only focus on interaction-induced theory.
. The main long-range contributions to the energy and electric properties of interacting molecules are as follows: electrostatic (multipole-multipole), polarization (induction and dispersion), resonance, relativistic, magnetic and retardation. The short-range interactions include the direct electrostatic, exchange, repulsion, change transfer effects. Note that the long-range interactions can be well described in an analytical form using classical electrodynamics for some approximation of the perturbation theory. In turn, for short distances the methods of quantum chemistry are only effective. In part of analytical theory for large R this book will only focus on interaction-induced theory.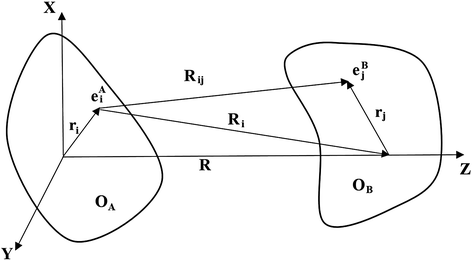
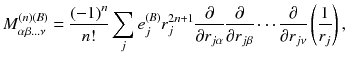
 take the values X, Y, Z (the number of Greek indexes equals to n ),
take the values X, Y, Z (the number of Greek indexes equals to n ), 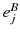 is any charge of the molecule B and
is any charge of the molecule B and 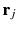 is the radius-vector of the charge relatively to the origin of local coordinate system OB. The multipole electrical moments in the form (2.1.1) appear when the interaction Hamiltonian is expanded in the Taylor series about the point OB (see Sect. ) if we put in it n = 0, 1 and 2 (analogously, for larger values of n the highest multipole moments can be written in the explicit form):
is the radius-vector of the charge relatively to the origin of local coordinate system OB. The multipole electrical moments in the form (2.1.1) appear when the interaction Hamiltonian is expanded in the Taylor series about the point OB (see Sect. ) if we put in it n = 0, 1 and 2 (analogously, for larger values of n the highest multipole moments can be written in the explicit form):