De Gruyter STEM
ISBN 9781501524608
e-ISBN (PDF) 9781501524615
e-ISBN (EPUB) 9781501516177
Bibliographic information published by the Deutsche Nationalbibliothek
The Deutsche Nationalbibliothek lists this publication in the Deutsche Nationalbibliografie; detailed bibliographic data are available on the Internet at http://dnb.dnb.de.
2021 Walter de Gruyter GmbH, Berlin/Boston
Symbols for physical constants and properties
Tab. 1: Symbols for physical constants and properties frequently used in this book.
| Quantity | Symbol | Value or typical units |
|---|
| Permittivity of free space | | 8.854 1012 F/m |
| Dielectric constant (relative permittivity) | r | Dimensionless |
| Unit charge | e | 1.602 1019 coulombs |
| Energy of an electronic state | E | eV |
| Fermi energy | EF | eV |
| Band gap energy | Eg | eV |
| Urbach energy | EU | meV |
| Density of states (DOS) | g(E) | cm3 |
| Reduced Planck constant | 6.582 1016 eVs |
| Boltzmann constant | kB | 8.617 105 eV/K |
| Mass of a free electron | me | 9.109 1031 kg |
| Effective mass of the charge carrier | m* | kg |
| Charge carrier mobility | m2/(Vs) |
| Charge carrier concentration | nc | cm3 |
| Sheet resistance | Rs | /sq |
| Electrical conductivity | S/cm |
| Absolute temperature | T | Kelvin |
Introduction
This book focuses on the fundamental interconnection between the nanostructure and the properties of conjugated polymers. The linear and rigid polymeric backbones that favor extended conjugation are prone to crystallization. In many cases, the conjugated polymers are semicrystalline. Their morphology results from nanoscale crystallites which are surrounded by an amorphous phase [).
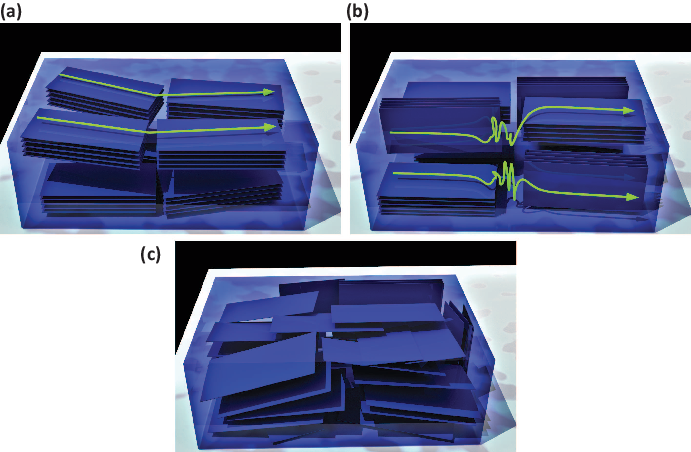
Fig. 1.1: Schematics of three possible nanocrystallite orientation distributions in semicrystalline conjugated polymers: (a) preferential face-on texture, (b) mixed face-on and edge-on texture, and (c) random orientation. The green lines with arrows suggest possible conformations for individual macromolecular chains.
In a semicrystalline conjugated polymer, individual chains are subjected to vastly different local environments. A chain can be confined entirely within a single nanocrystallite or segregate completely in the amorphous domain. Alternatively, part of the chain can reside in a nanocrystallite, while the remainder of the chain is part of the surrounding amorphous region. Additional possibilities arise for higher molecular weight chains. Of particular interest are tie chains, which fully span amorphous domains to interconnect two or more nanocrystallites. The electronic transport along the tie chains will be influenced by the relative orientation of the nanocrystals being connected.
Engineering order and orientation is essential for optimizing electronic transport in conjugated polymers. Undoped polymers can be useful as semiconductors. Through careful control over the nanoscale morphology and doping, metallic-like conduction can result. In addition, the nanoscale structure and texture of conjugated polymers underlie their optical, ionic, and thermal, transport properties.
Lightweight and mechanically compliant conjugated polymers have great potential for next generation flexible, mobile, and wearable devices. Semiconducting polymers are of interest for fabricating organic transistors, while semimetallic polymers are of interest for thermoelectric (Chapter 6) and spintronic (Chapter 7) applications [].
Combining the formal approaches of organic chemistry and solid-state physics is a powerful means to understand the charge transport mechanisms in semicrystalline conjugated polymers. The chemical perspective starts from atomic orbitals, which overlap to form extended molecular orbitals that delocalize electrons over multiple atoms. The solid-state physics approach starts from fully delocalized wavefunctions, known as Bloch waves, which give rise to electronic band structure. Upon the addition of disorder and defects, the extent of the wave function is reduced and can become partially localized over a subset of atoms.
This introductory chapter summarizes the chemistry- and physics-based descriptions of electronic conduction along a single isolated conjugated polymer chain. Subsequent chapters will focus on how the nanoscale morphology and orientation that develop when multiple conjugated polymer chains interact in the solid state impact electrical conduction as well as the transport of photons, phonons, and ions.
1.1 Chemical bonding and electronic states
The groundbreaking discovery of polymers that conduct electricity was recognized with Nobel Prize in Chemistry in 2000 to Hideki Shirakawa, Alan Heeger, and Alan MacDiarmid. Prior to this discovery, polymers were considered to be electrical insulators, having a band gap in excess of 5 eV between the valence and conduction bands. Such dielectric polymers have only sigma () chemical bonds, representing molecular orbitals which are spatially localized between pairs of atoms.
In addition to bonds, conjugated polymers possess bonds formed from the overlap of p-orbitals on adjacent atoms ( schematically shows the two molecular orbitals formed from the overlap of two pz-orbitals. The lower energy molecular orbital is the bonding state, formed by the constructive addition of the two atomic orbitals. The other is a higher energy * antibonding state resulting from the destructive (out-of-phase) addition of the two atomic orbitals. In the molecular ground state, both electrons fill the bonding state, leaving the antibonding state unoccupied.
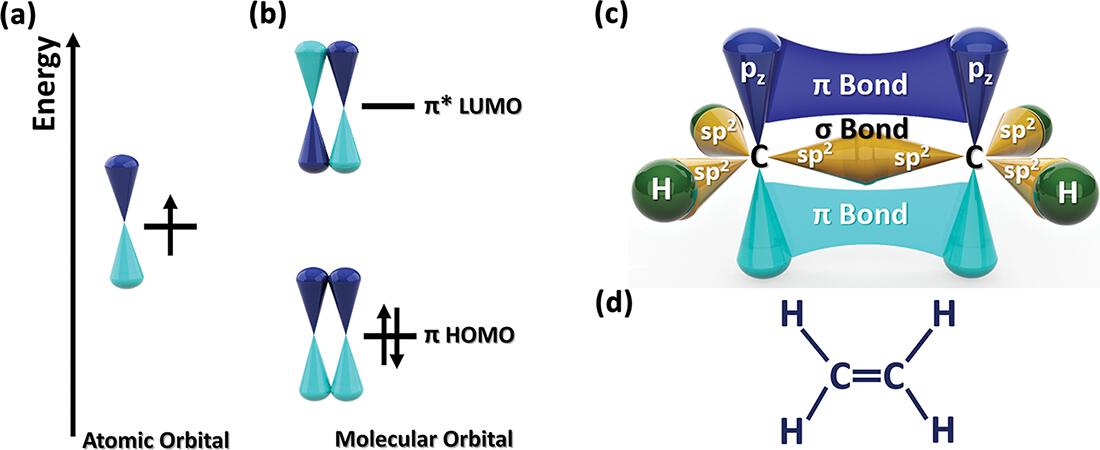
Fig. 1.2: Schematics of electronic orbitals and their associated ground state energy level diagrams: (a) an atomic p-orbital having two lobes of different phase, occupied by a single electron, and (b) the two molecular orbitals resulting from overlap p-orbitals on two adjacent atoms. The lower energy bonding state is fully occupied by two electrons, while the higher energy * antibonding state is unoccupied. For the molecule ethylene, (c) a schematic of the molecular orbitals, revealing both the and the bonding characteristics of the carboncarbon double bond, and (d) the formal chemical structure. Adapted with permission from Heydari Gharahcheshmeh and Gleason, Mater. Today Adv., 8, 10008 (2020) Copyright 2020, Elsevier.
A simple example of bonding occurs between the two carbon atoms in a molecule of ethylene, which has the chemical structure, H2C=CH2. The carboncarbon double bond consists of both and bonding components (). The bonding occurs between the sp2 hybridized orbitals of the two carbon atoms. The sp2 hybridization involves the s, px, and py atomic orbitals. The three resulting hybrid orbitals lie in the xy













