Answers to
Even-Numbered Problems
Page AP-1
Chapter 1
1.4 (a) Hypothesis. (b) Law. (c) Theory. 1.10 71.2 g. 1.12 (a) 41C. (b) 11.3F. (c) 1.1 10F. (d) 233C. 1.14 (a) 196C. (b) 269C. (c) 328C. 1.18 (a) 0.0152. (b) 0.0000000778. 1.20 (a) 1.8 10. (b) 1.14 10. (c) 5 10. (d) 1.3 10. 1.22 (a) One. (b) Three. (c) Three. (d) Four. (e) Two or three. (f ) One. (g) One or two. 1.24 (a) 1.28. (b) 3.18 10 mg. (c) 8.14 10 dm. (d) 3.8 m/s. 1.26 Tailor Xs measurements are the most precise. Tailor Ys measurements are the least accurate and least precise. Tailor Zs measurements are the most accurate. 1.28 (a) 1.10 10 mg. (b) 6.83 10 m. (c) 7.2 10 L. (d) 6.24 10 lb. 1.30 3.1557 10 s. 1.32 (a) 118 in/s. (b) 1.80 10 m/min. (c) 10.8 km/h. 1.34 178 mph. 1.36 3.7 10 g Pb. 1.38 (a) 1.5 10 lb. (b) 4.4 10 s. (c) 2.3 m. (d) 8.86 10 L. 1.40 6.25 10 g/cm. 1.42 27 y. 1.44 Float: 8 lb, 9 lb, 10 lb, 11 lb, 12 lb. 1.50 (a) Cs. (b) Ge. (c) Ga. (d) Sr. (e) U. (f ) Se. (g) Ne. (h) Cd. 1.52 (a) Homogeneous mixture. (b) Element. (c) Compound. (d) Homogeneous mixture. (e) Heterogeneous mixture. (f ) Heterogeneous mixture. (g) Element. 1.58 (a) Physical change. (b) Chemical change. (c) Physical change. (d) Chemical change. (e) Physical change. 1.60 (a) Chemical. (b) Chemical. (c) Physical. (d) Physical. (e) Chemical. 1.62 2.6 g/cm 1.64 9.20 cm. 1.66 767 mph. 1.68 Liquid must be less dense than ice; temperature below 0C. 1.70 2.3 10 cm. 1.72 6.4. 1.74 73S. 1.76 (a) 8.6 10 L air/day. (b) 0.018 L CO/day. 1.78 26,700,000 basketballs. 1.80 7.0 10 L. 1.82 88 lb; 40 kg. 1.84 O: 4.0 10 g; C: 1.1 10 g; H: 6.2 10 g; N: 2 10 g; Ca: 9.9 10 g; P: 7.4 10 g. 1.86 4.6 10C; 8.6 10F. 1.88 $2.4 10. 1.90 5.4 10 Fe atoms. 1.92 29 times. 1.94 1.450 10 mm. 1.96 1.3 10 mL. 1.98 (a) 11.063 mL. (b) 0.78900 g/mL. (c) 7.140 g/mL. 1.100 0.88 s. 1.102 (a) 327 L CO. (b) 5.0 10 g/L. (c) 1.20 10 g/mL. 1.104 0.85 cm. 1.106 4.97 10 g. 1.108 2.413 g/mL. 1.110 The glass bottle would crack.
Chapter 2
2.8 0.12 mi. 2.14 145. 2.16 N(7,8,7); S(16,17,16); Cu(29,34,29); Sr(38,46,38); Ba(56,74,56); W(74,112,74); Hg(80,122,80). 2.18 (a) 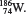
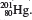 2.24 (a) Metallic character increases down a group. (b) Metallic character decreases from left to right. 2.26 F and Cl; Na and K; P and N. 2.32 (a) Diatomic molecule and compound. (b) Polyatomic molecule and compound. (c) Polyatomic molecule and element. 2.34 (a) H and F. (b) HCl and CO. (c) S and P. (d) HO and CHO (sucrose). 2.36 (protons, electrons): K + (19,18); Mg 2+ (12,10); Fe 3+ (26,23); Br(35,36); Mn 2+ (25,23); C(6,10); Cu 2+ (29,27). 2.38 (a)
2.24 (a) Metallic character increases down a group. (b) Metallic character decreases from left to right. 2.26 F and Cl; Na and K; P and N. 2.32 (a) Diatomic molecule and compound. (b) Polyatomic molecule and compound. (c) Polyatomic molecule and element. 2.34 (a) H and F. (b) HCl and CO. (c) S and P. (d) HO and CHO (sucrose). 2.36 (protons, electrons): K + (19,18); Mg 2+ (12,10); Fe 3+ (26,23); Br(35,36); Mn 2+ (25,23); C(6,10); Cu 2+ (29,27). 2.38 (a) 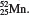
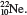 (c)
(c) 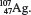 (d)
(d) 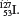 (e) Pu. 2.46 (a) CuBr. (b) MnO. (c) HgI. (d) Mg(PO). 2.48 (a) AlBr. (b) NaSO. (c) NO. (d) KCrO. 2.50 CHO. 2.52 Ionic: NaBr, BaF, CsCl. Molecular: CH, CCl, ICl, NF. 2.60 (a) Potassium hypochlorite. (b) Silver carbonate. (c) Iron(II) chloride. (d) Potassium permanganate. (e) Cesium chlorate. (f ) Hypoiodous acid. (g) Iron(II) oxide. (h) Iron(III) oxide. (i) Titanium(IV) chloride. ( j) Sodium hydride. (k) Lithium nitride. (l) Sodium oxide. (m) Sodium peroxide. (n) Iron(III) chloride hexahydrate. 2.62 (a) CuCN. (b) Sr(ClO). (c) HBrO. (d) HI( aq ). (e) Na(NH)PO. (f ) PbCO. (g) SnF. (h) PS. (i) HgO. ( j) HgI. (k) SeF. 2.64 (a) Dinitrogen pentoxide (NO). (b) Boron trifluoride (BF). (c) Dialuminum hexabromide (AlBr). 2.66 (c) Changing the electrical charge of an atom usually has a major effect on its chemical properties. 2.68 I. 2.70 NaCl is an ionic compound. It does not form molecules. 2.72 Element: (b), (c), (e), (f ), (g), ( j), (k). Molecules but not compounds: (b), (f ), (g), (k). Compounds but not molecules: (i), (l). Compounds and molecules: (a), (d), (h). 2.74 (a) Ne: 10 p, 10 n. (b) Cu: 29 p, 34 n. (c) Ag: 47 p, 60 n. (d) W: 74 p, 108 n. (e) Po: 84 p, 119 n. (f ) Pu: 94 p, 140 n. 2.76 (a) Cu. (b) P. (c) Kr. (d) Cs. (e) Al. (f ) Sb. (g) Cl. (h) Sr. 2.78 (a) The magnitude of a particle scattering depends on the number of protons present. (b) Density of nucleus: 3.25 10 g/cm; density of space occupied by electrons: 3.72 10 g/cm. The result supports Rutherfords model. 2.80 The empirical and molecular formulas of acetaminophen are both CHNO. 2.82 (a) Tin(IV) chloride. (b) Copper(I) oxide. (c) Cobalt(II) nitrate. (d) Sodium dichromate. 2.84 (a) Ionic compounds formed between metallic and nonmetallic elements. (b) Transition metals, lanthanides, and actinides. 2.86 Na. 2.88 Hg and Br. 2.90 H, N, O, F, Cl, He, Ne, Ar, Kr, Xe, Rn. 2.92 Unreactive. He, Ne, and Ar are chemically inert. 2.94 Ra is a radioactive decay product of U-238. 2.96 Se. 2.98 (a) NaH, sodium hydride. (b) BO, diboron trioxide. (c) NaS, sodium sulfide. (d) AlF, aluminum fluoride. (e) OF, oxygen difluoride. (f ) SrCl, strontium chloride. 2.100 NF (nitrogen trifluoride), PBr (phosphorus pentabromide), SCl (sulfur dichloride). 2.102 1st row: Mg 2+ ,
(e) Pu. 2.46 (a) CuBr. (b) MnO. (c) HgI. (d) Mg(PO). 2.48 (a) AlBr. (b) NaSO. (c) NO. (d) KCrO. 2.50 CHO. 2.52 Ionic: NaBr, BaF, CsCl. Molecular: CH, CCl, ICl, NF. 2.60 (a) Potassium hypochlorite. (b) Silver carbonate. (c) Iron(II) chloride. (d) Potassium permanganate. (e) Cesium chlorate. (f ) Hypoiodous acid. (g) Iron(II) oxide. (h) Iron(III) oxide. (i) Titanium(IV) chloride. ( j) Sodium hydride. (k) Lithium nitride. (l) Sodium oxide. (m) Sodium peroxide. (n) Iron(III) chloride hexahydrate. 2.62 (a) CuCN. (b) Sr(ClO). (c) HBrO. (d) HI( aq ). (e) Na(NH)PO. (f ) PbCO. (g) SnF. (h) PS. (i) HgO. ( j) HgI. (k) SeF. 2.64 (a) Dinitrogen pentoxide (NO). (b) Boron trifluoride (BF). (c) Dialuminum hexabromide (AlBr). 2.66 (c) Changing the electrical charge of an atom usually has a major effect on its chemical properties. 2.68 I. 2.70 NaCl is an ionic compound. It does not form molecules. 2.72 Element: (b), (c), (e), (f ), (g), ( j), (k). Molecules but not compounds: (b), (f ), (g), (k). Compounds but not molecules: (i), (l). Compounds and molecules: (a), (d), (h). 2.74 (a) Ne: 10 p, 10 n. (b) Cu: 29 p, 34 n. (c) Ag: 47 p, 60 n. (d) W: 74 p, 108 n. (e) Po: 84 p, 119 n. (f ) Pu: 94 p, 140 n. 2.76 (a) Cu. (b) P. (c) Kr. (d) Cs. (e) Al. (f ) Sb. (g) Cl. (h) Sr. 2.78 (a) The magnitude of a particle scattering depends on the number of protons present. (b) Density of nucleus: 3.25 10 g/cm; density of space occupied by electrons: 3.72 10 g/cm. The result supports Rutherfords model. 2.80 The empirical and molecular formulas of acetaminophen are both CHNO. 2.82 (a) Tin(IV) chloride. (b) Copper(I) oxide. (c) Cobalt(II) nitrate. (d) Sodium dichromate. 2.84 (a) Ionic compounds formed between metallic and nonmetallic elements. (b) Transition metals, lanthanides, and actinides. 2.86 Na. 2.88 Hg and Br. 2.90 H, N, O, F, Cl, He, Ne, Ar, Kr, Xe, Rn. 2.92 Unreactive. He, Ne, and Ar are chemically inert. 2.94 Ra is a radioactive decay product of U-238. 2.96 Se. 2.98 (a) NaH, sodium hydride. (b) BO, diboron trioxide. (c) NaS, sodium sulfide. (d) AlF, aluminum fluoride. (e) OF, oxygen difluoride. (f ) SrCl, strontium chloride. 2.100 NF (nitrogen trifluoride), PBr (phosphorus pentabromide), SCl (sulfur dichloride). 2.102 1st row: Mg 2+ , 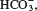 Mg(HCO). 2nd row: Sr 2+ , Cl, strontium chloride. 3rd row: Fe(NO), iron(III) nitrite. 4th row: Mn 2+ ,
Mg(HCO). 2nd row: Sr 2+ , Cl, strontium chloride. 3rd row: Fe(NO), iron(III) nitrite. 4th row: Mn 2+ ,  Mn(ClO). 5th row: Sn 4+ , Br, tin(IV) bromide. 6th row: Co(PO), cobalt(II) phosphate. 7th row: HgI, mercury(I) iodide. 8th row: Cu + ,
Mn(ClO). 5th row: Sn 4+ , Br, tin(IV) bromide. 6th row: Co(PO), cobalt(II) phosphate. 7th row: HgI, mercury(I) iodide. 8th row: Cu + , 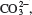 copper(I) carbonate. 9th row: Li + , N, LiN. 10th row: AlS, aluminum sulfide. 2.104 1.91 10 g. Mass is too small to be detected. 2.106 (a) Volume of a sphere is given by V = (4/3) r . Volume is also proportional to the number of neutrons and protons present, or the mass number A . Therefore, r A or r A 1/3 . (b) 5.1 10 m. (c) The nucleus occupies only 3.5 10% of the atoms volume. The result supports Rutherfords model. 2.108 (a) Yes. (b) Ethane: CH and CH. Acetylene: CH and CH. 2.110 Manganese (Mn). 2.112 From left to right: chloric acid, nitrous acid, hydrocyanic acid, and sulfuric acid. 2.114 XY. X is likely in Group 4 or Group 14 and Y is likely in Group 16. Examples: titanium(IV) oxide (TiO), tin(IV) oxide (SnO), and lead(IV) oxide (PbO).
copper(I) carbonate. 9th row: Li + , N, LiN. 10th row: AlS, aluminum sulfide. 2.104 1.91 10 g. Mass is too small to be detected. 2.106 (a) Volume of a sphere is given by V = (4/3) r . Volume is also proportional to the number of neutrons and protons present, or the mass number A . Therefore, r A or r A 1/3 . (b) 5.1 10 m. (c) The nucleus occupies only 3.5 10% of the atoms volume. The result supports Rutherfords model. 2.108 (a) Yes. (b) Ethane: CH and CH. Acetylene: CH and CH. 2.110 Manganese (Mn). 2.112 From left to right: chloric acid, nitrous acid, hydrocyanic acid, and sulfuric acid. 2.114 XY. X is likely in Group 4 or Group 14 and Y is likely in Group 16. Examples: titanium(IV) oxide (TiO), tin(IV) oxide (SnO), and lead(IV) oxide (PbO).
Chapter 3
3.6 7.5% and 92.5%. 3.8 5.1 10 amu. 3.12 5.8 10 light-yr. 3.14 9.96 10 mol Co. 3.16 3.01 10 g Au. 3.18 (a) 1.244 10 g/As atom. (b) 9.746 10 g/Ni atom. 3.20 6.0 10 Cu atoms. 3.22 Pb. 3.24 (a) 73.89 g. (b) 76.15 g. (c) 119.37 g. (d) 176.12 g. (e) 101.11 g. (f ) 100.95 g. 3.26 6.69 10 CH molecules. 3.28 C: 1.10 10 atoms; S: 5.50 10 atoms; H: 3.30 10 atoms; O: 5.50 10 atoms. 3.30 8.56 10 molecules. 3.34 7. 3.38 C: 10.06%; H: 0.8442%; Cl: 89.07%. 3.40 (d) Ammonia, NH. 3.42 5.97 g F. 3.44 (a) CHO. (b) KCN. 3.48 CH. 3.50 CHNO. 3.52 CHONNa. 3.58 (a) 2NO 2NO + O. (b) 2KNO 2KNO + O. (c) NHNO NO + 2HO. (d) NHNO N + 2HO. (e) 2NaHCO NaCO + HO + CO. (f ) PO + 6HO 4HPO. (g) 2HCl + CaCO CaCl + HO + CO. (h) 2Al + 3HSO Al(SO) + 3H. (i) CO + 2KOH KCO + HO. ( j) CH + 2O CO + 2HO. (k) BeC + 4HO 2Be(OH) + CH. (l) 3Cu + 8HNO 3Cu(NO) + 2NO + 4HO. (m) S + 6HNO HSO + 6NO + 2HO. (n) 2NH + 3CuO 3Cu + N + 3HO. 3.62 (d). 3.64 1.01 mol. 3.66 20 mol. 3.68 39.3 g S. 3.70 (a) 2NaHCO NaCO + CO + HO. (b) 78.3 g. 3.72 255.9 g; 0.324 L. 3.74 0.294 mol. 3.76 (a) NHNO NO + 2HO. (b) 20 g. 3.78 18.0 g. 3.82 1 mole H left and 6 moles NH produced. 3.84 (a) 2NH + HSO (NH)SO. (b) 5.23 g NH; 21.0 g HSO. 3.86 HCl; 23.4 g. 3.90 (a) 7.05 g. (b) 92.9%. 3.92 3.48 10 g. 3.94 8.55 g; 76.6%. 3.96 100%. 3.98 Rb: 72.1%; Rb: 27.9%. 3.100 (b). 3.102 (a) CH + 8O 5CO + 6HO. (b) NaHCO + HCl CO + NaCl + HO. (c) 6Li + N 2LiN. (d) PCl + 3HO HPO + 3HCl. (e) 3CuO + 2NH 3Cu + N + 3HO. 3.104 ClO. 3.106 18.7 g. 3.108 (a) 0.212 mol. (b) 0.424 mol. 3.110 18. 3.112 2.4 10 atoms. 3.114 65.4 amu; Zn. 3.116 89.5%. 3.118 CHO; CHO. 3.120 51.9 g/mol; Cr. 3.122 29.54%. 3.124 1.6 10 g/mol. 3.126 NaBr: 24.03%; NaSO: 75.97%. 3.128 CH + 5O 3CO + 4HO. 3.130 Ca: 38.76%; P: 19.97%; O: 41.27%. 3.132 Yes. 3.134 2.01 10 molecules. 3.136 16.00 amu. 3.138 (e). 3.140 PtCl; PtCl. 3.142 (a) 12 g; 28 mL. (b) 15 g. 3.144 (a) X: MnO; Y: MnO. (b) 3MnO MnO + O. 3.146 6.1 10 tons. 3.148 CHClFO. C: 19.53%; H: 1.093%; Cl: 19.21%; F: 51.49%; O: 8.672%. 3.150 MgN (magnesium nitride). 3.152 PbCH. 3.154 (a) 4.3 10 atoms. (b) 1.6 10 pm. 3.156 28.97 g/mol. 3.158 (a) FeO + 6HCl 2FeCl + 3HO. (b) 396 g FeCl. 3.160 (a) CH + 3HO 3CO + 7H. (b) 9.09 10 kg. 3.162 (a) There is only one reactant so the use of limiting reagent is unnecessary. (b) The term limiting reagent usually applies only to one reactant. 3.164 (a) $0.47/kg. (b) 0.631 kg KO. 3.166 BaBr. 3.168 NaCl: 32.17%; NaSO: 20.09%; NaNO: 47.75%.












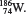
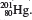 2.24 (a) Metallic character increases down a group. (b) Metallic character decreases from left to right. 2.26 F and Cl; Na and K; P and N. 2.32 (a) Diatomic molecule and compound. (b) Polyatomic molecule and compound. (c) Polyatomic molecule and element. 2.34 (a) H and F. (b) HCl and CO. (c) S and P. (d) HO and CHO (sucrose). 2.36 (protons, electrons): K + (19,18); Mg 2+ (12,10); Fe 3+ (26,23); Br(35,36); Mn 2+ (25,23); C(6,10); Cu 2+ (29,27). 2.38 (a)
2.24 (a) Metallic character increases down a group. (b) Metallic character decreases from left to right. 2.26 F and Cl; Na and K; P and N. 2.32 (a) Diatomic molecule and compound. (b) Polyatomic molecule and compound. (c) Polyatomic molecule and element. 2.34 (a) H and F. (b) HCl and CO. (c) S and P. (d) HO and CHO (sucrose). 2.36 (protons, electrons): K + (19,18); Mg 2+ (12,10); Fe 3+ (26,23); Br(35,36); Mn 2+ (25,23); C(6,10); Cu 2+ (29,27). 2.38 (a) 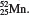
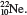 (c)
(c) 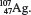 (d)
(d) 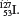 (e) Pu. 2.46 (a) CuBr. (b) MnO. (c) HgI. (d) Mg(PO). 2.48 (a) AlBr. (b) NaSO. (c) NO. (d) KCrO. 2.50 CHO. 2.52 Ionic: NaBr, BaF, CsCl. Molecular: CH, CCl, ICl, NF. 2.60 (a) Potassium hypochlorite. (b) Silver carbonate. (c) Iron(II) chloride. (d) Potassium permanganate. (e) Cesium chlorate. (f ) Hypoiodous acid. (g) Iron(II) oxide. (h) Iron(III) oxide. (i) Titanium(IV) chloride. ( j) Sodium hydride. (k) Lithium nitride. (l) Sodium oxide. (m) Sodium peroxide. (n) Iron(III) chloride hexahydrate. 2.62 (a) CuCN. (b) Sr(ClO). (c) HBrO. (d) HI( aq ). (e) Na(NH)PO. (f ) PbCO. (g) SnF. (h) PS. (i) HgO. ( j) HgI. (k) SeF. 2.64 (a) Dinitrogen pentoxide (NO). (b) Boron trifluoride (BF). (c) Dialuminum hexabromide (AlBr). 2.66 (c) Changing the electrical charge of an atom usually has a major effect on its chemical properties. 2.68 I. 2.70 NaCl is an ionic compound. It does not form molecules. 2.72 Element: (b), (c), (e), (f ), (g), ( j), (k). Molecules but not compounds: (b), (f ), (g), (k). Compounds but not molecules: (i), (l). Compounds and molecules: (a), (d), (h). 2.74 (a) Ne: 10 p, 10 n. (b) Cu: 29 p, 34 n. (c) Ag: 47 p, 60 n. (d) W: 74 p, 108 n. (e) Po: 84 p, 119 n. (f ) Pu: 94 p, 140 n. 2.76 (a) Cu. (b) P. (c) Kr. (d) Cs. (e) Al. (f ) Sb. (g) Cl. (h) Sr. 2.78 (a) The magnitude of a particle scattering depends on the number of protons present. (b) Density of nucleus: 3.25 10 g/cm; density of space occupied by electrons: 3.72 10 g/cm. The result supports Rutherfords model. 2.80 The empirical and molecular formulas of acetaminophen are both CHNO. 2.82 (a) Tin(IV) chloride. (b) Copper(I) oxide. (c) Cobalt(II) nitrate. (d) Sodium dichromate. 2.84 (a) Ionic compounds formed between metallic and nonmetallic elements. (b) Transition metals, lanthanides, and actinides. 2.86 Na. 2.88 Hg and Br. 2.90 H, N, O, F, Cl, He, Ne, Ar, Kr, Xe, Rn. 2.92 Unreactive. He, Ne, and Ar are chemically inert. 2.94 Ra is a radioactive decay product of U-238. 2.96 Se. 2.98 (a) NaH, sodium hydride. (b) BO, diboron trioxide. (c) NaS, sodium sulfide. (d) AlF, aluminum fluoride. (e) OF, oxygen difluoride. (f ) SrCl, strontium chloride. 2.100 NF (nitrogen trifluoride), PBr (phosphorus pentabromide), SCl (sulfur dichloride). 2.102 1st row: Mg 2+ ,
(e) Pu. 2.46 (a) CuBr. (b) MnO. (c) HgI. (d) Mg(PO). 2.48 (a) AlBr. (b) NaSO. (c) NO. (d) KCrO. 2.50 CHO. 2.52 Ionic: NaBr, BaF, CsCl. Molecular: CH, CCl, ICl, NF. 2.60 (a) Potassium hypochlorite. (b) Silver carbonate. (c) Iron(II) chloride. (d) Potassium permanganate. (e) Cesium chlorate. (f ) Hypoiodous acid. (g) Iron(II) oxide. (h) Iron(III) oxide. (i) Titanium(IV) chloride. ( j) Sodium hydride. (k) Lithium nitride. (l) Sodium oxide. (m) Sodium peroxide. (n) Iron(III) chloride hexahydrate. 2.62 (a) CuCN. (b) Sr(ClO). (c) HBrO. (d) HI( aq ). (e) Na(NH)PO. (f ) PbCO. (g) SnF. (h) PS. (i) HgO. ( j) HgI. (k) SeF. 2.64 (a) Dinitrogen pentoxide (NO). (b) Boron trifluoride (BF). (c) Dialuminum hexabromide (AlBr). 2.66 (c) Changing the electrical charge of an atom usually has a major effect on its chemical properties. 2.68 I. 2.70 NaCl is an ionic compound. It does not form molecules. 2.72 Element: (b), (c), (e), (f ), (g), ( j), (k). Molecules but not compounds: (b), (f ), (g), (k). Compounds but not molecules: (i), (l). Compounds and molecules: (a), (d), (h). 2.74 (a) Ne: 10 p, 10 n. (b) Cu: 29 p, 34 n. (c) Ag: 47 p, 60 n. (d) W: 74 p, 108 n. (e) Po: 84 p, 119 n. (f ) Pu: 94 p, 140 n. 2.76 (a) Cu. (b) P. (c) Kr. (d) Cs. (e) Al. (f ) Sb. (g) Cl. (h) Sr. 2.78 (a) The magnitude of a particle scattering depends on the number of protons present. (b) Density of nucleus: 3.25 10 g/cm; density of space occupied by electrons: 3.72 10 g/cm. The result supports Rutherfords model. 2.80 The empirical and molecular formulas of acetaminophen are both CHNO. 2.82 (a) Tin(IV) chloride. (b) Copper(I) oxide. (c) Cobalt(II) nitrate. (d) Sodium dichromate. 2.84 (a) Ionic compounds formed between metallic and nonmetallic elements. (b) Transition metals, lanthanides, and actinides. 2.86 Na. 2.88 Hg and Br. 2.90 H, N, O, F, Cl, He, Ne, Ar, Kr, Xe, Rn. 2.92 Unreactive. He, Ne, and Ar are chemically inert. 2.94 Ra is a radioactive decay product of U-238. 2.96 Se. 2.98 (a) NaH, sodium hydride. (b) BO, diboron trioxide. (c) NaS, sodium sulfide. (d) AlF, aluminum fluoride. (e) OF, oxygen difluoride. (f ) SrCl, strontium chloride. 2.100 NF (nitrogen trifluoride), PBr (phosphorus pentabromide), SCl (sulfur dichloride). 2.102 1st row: Mg 2+ , 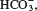 Mg(HCO). 2nd row: Sr 2+ , Cl, strontium chloride. 3rd row: Fe(NO), iron(III) nitrite. 4th row: Mn 2+ ,
Mg(HCO). 2nd row: Sr 2+ , Cl, strontium chloride. 3rd row: Fe(NO), iron(III) nitrite. 4th row: Mn 2+ ,  Mn(ClO). 5th row: Sn 4+ , Br, tin(IV) bromide. 6th row: Co(PO), cobalt(II) phosphate. 7th row: HgI, mercury(I) iodide. 8th row: Cu + ,
Mn(ClO). 5th row: Sn 4+ , Br, tin(IV) bromide. 6th row: Co(PO), cobalt(II) phosphate. 7th row: HgI, mercury(I) iodide. 8th row: Cu + , 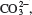 copper(I) carbonate. 9th row: Li + , N, LiN. 10th row: AlS, aluminum sulfide. 2.104 1.91 10 g. Mass is too small to be detected. 2.106 (a) Volume of a sphere is given by V = (4/3) r . Volume is also proportional to the number of neutrons and protons present, or the mass number A . Therefore, r A or r A 1/3 . (b) 5.1 10 m. (c) The nucleus occupies only 3.5 10% of the atoms volume. The result supports Rutherfords model. 2.108 (a) Yes. (b) Ethane: CH and CH. Acetylene: CH and CH. 2.110 Manganese (Mn). 2.112 From left to right: chloric acid, nitrous acid, hydrocyanic acid, and sulfuric acid. 2.114 XY. X is likely in Group 4 or Group 14 and Y is likely in Group 16. Examples: titanium(IV) oxide (TiO), tin(IV) oxide (SnO), and lead(IV) oxide (PbO).
copper(I) carbonate. 9th row: Li + , N, LiN. 10th row: AlS, aluminum sulfide. 2.104 1.91 10 g. Mass is too small to be detected. 2.106 (a) Volume of a sphere is given by V = (4/3) r . Volume is also proportional to the number of neutrons and protons present, or the mass number A . Therefore, r A or r A 1/3 . (b) 5.1 10 m. (c) The nucleus occupies only 3.5 10% of the atoms volume. The result supports Rutherfords model. 2.108 (a) Yes. (b) Ethane: CH and CH. Acetylene: CH and CH. 2.110 Manganese (Mn). 2.112 From left to right: chloric acid, nitrous acid, hydrocyanic acid, and sulfuric acid. 2.114 XY. X is likely in Group 4 or Group 14 and Y is likely in Group 16. Examples: titanium(IV) oxide (TiO), tin(IV) oxide (SnO), and lead(IV) oxide (PbO).