Science Fair Projects Using Hair Gel, Soda Bottles, and Slimy Stuff
Do all polymers melt? What does a chain of polymer atoms look like? Which cups insulate hot drinks best? Using easy-to-find materials and the scientific method, student scientists can learn the answers to these questions and more. For students interested in competing in science fairs, the book contains lots of great suggestions and ideas for further experiments.
ABOUT THE AUTHOR
Madeline Goodstein is a retired professor of chemistry from Central Connecticut State University. She is the author of numerous science project books with Enslow Publishers, Inc.

The author thanks Valerie A. Wilcox, Executive Director of the National Plastics Center and Museum, for arranging a guided tour, and Anne-Marie Arnold, In-House Education Director, for giving the tour of the museum.

Image Credit: Shutterstock
Protected by a helmet and goggles made of plastics, a freestyle motocross competitor performs a daring stunt.
What comes to mind when you think of plastics? How about wash-and-wear clothing that doesnt need ironing; bicycle and motorcycle helmets that save lives; thin, clear wrap to keep food moist and safe; automobile tires good for sixty thousand miles; bullet-proof vests; picnic cups that keep hot drinks hot and cold drinks cold; glue so instant that you have to be careful not to glue your fingers together; CDs and cassettes with your favorite music; carpets where food spills can be washed right off; and pot handles, quick-drying paints, chair and couch upholstery, airplane windows, tabletops and countertops, foam mattresses, patio chairs, boat hulls, vinyl floor tiles, dishes, toys, bathtub caulk, plumbing pipes, fences, insulation, surgical gowns, computer chips, and much more. As you can see, plastics are used for many things in our lives.
How do you know when something is plastic? Often, you can tell that something is plastic when you lift it. This is because many plastics are lightweight. The feel of the plastic is often another way to tell. A chunk of plastic feels firm yet not as hard as metal. Plastic wraps have their own special properties. They are soft and flexible. A plastic wrap sticks to itself and can be stretched.
Weight, feel, and stretchability are all special characteristics of plastics that are unlike those of other materials. Why do plastics have these properties? The answers to this question will be explored in the experiments in this book.
What is a plastic? What makes it different from other materials? The answer is a chemical one. All plastics are made of a special kind of chemical. This special kind of chemical is called a polymer. Plastics are all made principally of polymers. A polymer is a very large molecule. A polymer molecule may be thousands or even millions of times larger than a non-polymer molecule.
For any substance, the smallest bit of matter that can be identified as that substance is called a molecule. An example of a molecule that we all know is the water molecule, H2O. The tiny particles that together make up a cup or a pond or a lake of water are each made up of two hydrogen (H) atoms and one oxygen (O) atom. Each H2O particle is a molecule, the smallest bit of water possible.
Until polymers were discovered, it was believed that all molecules were made up of small numbers of atoms. The discovery of polymers changed that belief. Now, we know that polymer molecules are made up of hundreds, thousands, or even hundreds of thousands of atoms in long chains or networks (chains connected to each other at various spots).
Does the extreme length of the polymer molecule give it properties very different from that of small molecules? The answer to that is a resounding yes! The length of a polymer molecule accounts for the distinctive behaviors of the plastics they make.
The long chains are formed from small molecules called monomers. Monomers combine end to end in a chemical reaction to form the polymer chain. Imagine a group of identical boys gathered in a school gymnasium: . The long chain is the polymer. Each boy is a monomer. The process of polymer formation from monomers is called polymerization. Many chains may be formed at the same time, and the chains may even join together to form a network.
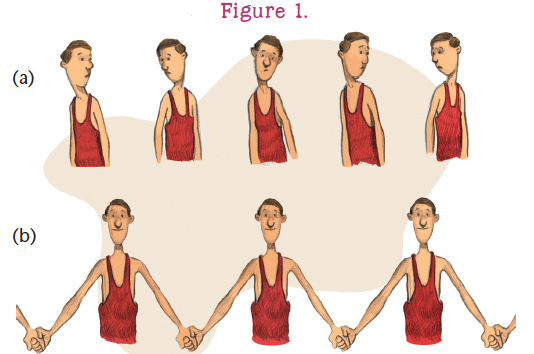
: a) In this model, each boy represents a monomer.
b) When the boys join hands, they form a polymer.
A polymer may also form from two different monomers. For example, the gymnasium might hold a large number of boys and girls. At a signal, one boy is required to clasp a girls hand. Holding on to the first boy, the girl clasps the hand of another boy with her other hand. Holding on, that boy clasps another girls hand and so on until a long chain is formed. The boys and girls represent monomers that bond together to form the repeat segment. The chain of repeat segments is a polymer, as shown in . Again, many chains may form, and they may join together to form a network.
All of the wonderful man-made plastic products that improve our lives today are based on polymers. Usually, plastics contain more than just the polymer or polymers. Additives are introduced to make the polymer more flexible or more heat-resistant or to improve whatever special quality is needed to make it useful.
If all the plastics in the world suddenly disappeared, you might find yourself standing naked on a dirt floor. If all the natural polymers disappeared, you wouldnt exist! Proteins, certain sugars, DNA, and RNAall of which help to make up our bodiesare natural polymers. Plants would not exist either, because the wall of a plant cell is built of cellulose, a natural polymer. The starch that helps to make up plants is also a natural polymer.
This book has many experiments designed to illustrate how the length and structure of a polymer chain cause it to have special properties. By doing these experiments, you can get started learning about what causes gooey, bouncy, stretchy, rubbery, hard, flexible, or glassy plastics.
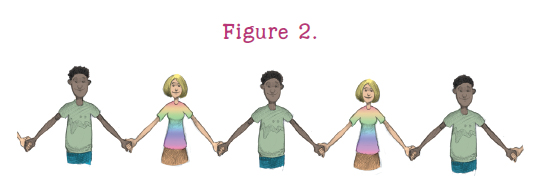
: In this model of a polymer, each boy represents one specific monomer and each girl stands for a different monomer. The monomers join together in a chemical reaction to form a boy-girl sequence that repeats for the entire length of the polymer chain. A combination of boy-girl-girl would produce a different polymer.
Why have polymers become so widely used? In less than a century, they have replaced wood, metal, marble, fabric, paint, caulking, glue, and other products in many places. A major reason for their popularity is that plastics are easy to shape. Polymers can be molded into desired forms, drawn into fibers (threads), stretched, and/or bent. Also, the raw materials that make them are easy to obtain and often inexpensive. Plastics are usually lightweight and not damaged by chemicals. Many polymers are waterproof. Some plastics are such good electrical insulators that they are used to coat electrical wiring. It is no wonder there are so many uses for plastics.