Copyright 2014 by McGraw-Hill Education. All rights reserved. Except as permitted under the United States Copyright Act of 1976, no part of this publication may be reproduced or distributed in any form or by any means, or stored in a data base or retrieval system, without the prior written permission of the publisher. ISBN: 978-0-07-176789-7 MHID: 0-07-176789-4 The material in this eBook also appears in the print version of this title: ISBN: 978-0-07-176788-0, MHID: 0-07-176788-6. eBook conversion by codeMantra
Version 1.0 All trademarks are trademarks of their respective owners. Rather than put a trademark symbol after every occurrence of a trademarked name, we use names in an editorial fashion only, and to the benefit of the trademark owner, with no intention of infringement of the trademark.
Where such designations appear in this book, they have been printed with initial caps. McGraw-Hill Education eBooks are available at special quantity discounts to use as premiums and sales promotions or for use in corporate training programs. To contact a representative, please visit the Contact Us page at www.mhprofessional.com. TERMS OF USE This is a copyrighted work and McGraw-Hill Education and its licensors reserve all rights in and to the work. Use of this work is subject to these terms. Except as permitted under the Copyright Act of 1976 and the right to store and retrieve one copy of the work, you may not decompile, disassemble, reverse engineer, reproduce, modify, create derivative works based upon, transmit, distribute, disseminate, sell, publish or sublicense the work or any part of it without McGraw-Hill Educations prior consent.
You may use the work for your own noncommercial and personal use; any other use of the work is strictly prohibited. Your right to use the work may be terminated if you fail to comply with these terms. THE WORK IS PROVIDED AS IS. MCGRAW-HILL EDUCATION AND ITS LICENSORS MAKE NO GUARANTEES OR WARRANTIES AS TO THE ACCURACY, ADEQUACY OR COMPLETENESS OF OR RESULTS TO BE OBTAINED FROM USING THE WORK, INCLUDING ANY INFORMATION THAT CAN BE ACCESSED THROUGH THE WORK VIA HYPERLINK OR OTHERWISE, AND EXPRESSLY DISCLAIM ANY WARRANTY, EXPRESS OR IMPLIED, INCLUDING BUT NOT LIMITED TO IMPLIED WARRANTIES OF MERCHANTABILITY OR FITNESS FOR A PARTICULAR PURPOSE. McGraw-Hill Education and its licensors do not warrant or guarantee that the functions contained in the work will meet your requirements or that its operation will be uninterrupted or error free. Neither McGraw-Hill Education nor its licensors shall be liable to you or anyone else for any inaccuracy, error or omission, regardless of cause, in the work or for any damages resulting therefrom.
McGraw-Hill Education has no responsibility for the content of any information accessed through the work. Under no circumstances shall McGraw-Hill Education and/or its licensors be liable for any indirect, incidental, special, punitive, consequential or similar damages that result from the use of or inability to use the work, even if any of them has been advised of the possibility of such damages. This limitation of liability shall apply to any claim or cause whatsoever whether such claim or cause arises in contract, tort or otherwise.
Chemistry is a fascinating subject, but with all the material to be covered in any chemistry course it can be overwhelming. There are several types of chemistryinorganic chemistry, biochemistry, and organic chemistry, for examplebut all have a similar foundation.
Easy Chemistry Step-by-Step allows you to move at your own pace through basic chemistry concepts.
Some concepts will be easier than others to master. Each chapter begins with a foundation section that provides an introduction to the topic. This is followed by further, in-depth explanations. Step-by-step problem-solving strategies are provided to help build a solid foundation of chemical concepts. These strategies include questions to ask yourself while performing a skill and then examples for you to solve. Common mistakes are pointed out as missteps to be aware of.
The in-depth examples are followed by guided practice and exercises to try on your own. Although the chapters build on each other, they also stand alone so you can return to any particular concept and review it as needed. Learning chemistry is similar to learning a foreign language. You will learn the language of several types of compounds and their different naming systems. A solid foundation is important as you continue to take additional courses. Understanding the fundamentals of chemistry also requires knowledge of mathematics and how the mathematics relate to chemical concepts.
The goal of Easy Chemistry Step-by-Step is to provide the necessary foundation in chemical concepts and the mathematics of chemistry for you to be successful in your first chemistry course.
The Elements on the Periodic Table

Elements and compounds have names. The names vary by language but the symbol and formula of the element or compound remain the same and are controlled by an international committee (IUPAC). Some examples include
magnesio in Spanish, which is
magnesium in English, yet both are the symbol Mg;
argento in Italian,
plata in Spanish, and
silver in English all have the symbol Ag; and
crypto in Italian and
krypton in English both have the symbol Kr. To learn chemistry, you must first know the chemistry language of the elements!
Element Names and Location on the Periodic Table
The periodic table shows the arrangement of the elements. Learning the symbols and their names will take practice.
For many elements, the name and symbol are easy to learn because the name and symbol are similar to each other. There is no reasoning to finding an element on the periodic table, such as going in alphabetical order. One way to help find an element is to know if the element is a metal or nonmetal. Metals are on the left of the table on the next page and nonmetals are on the far right. 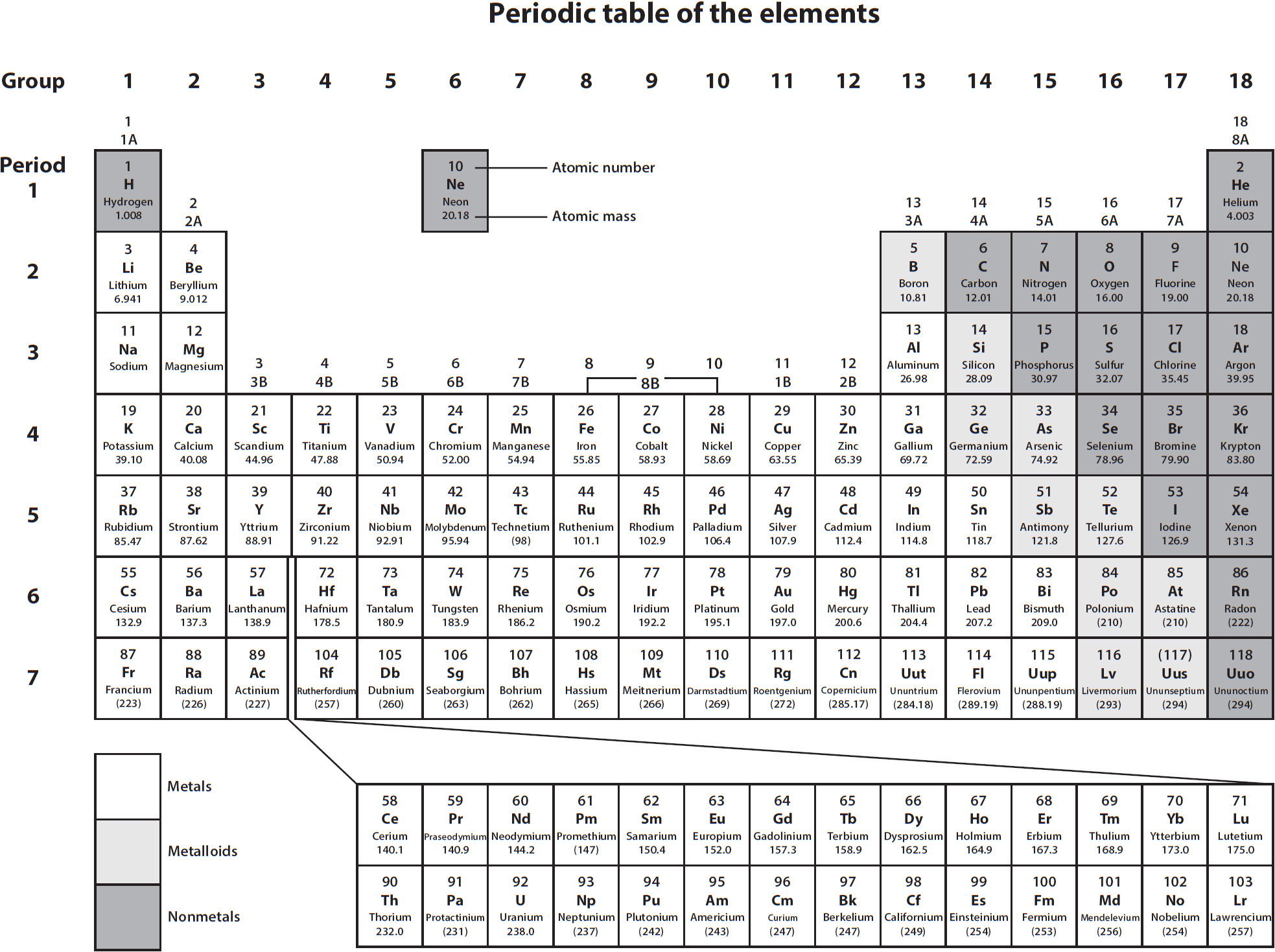
Questions to Ask Yourself
 Step 1.
Step 1. Is the element or symbol a metal? Remember to look on the left-hand side of the table.
Problems It is important to know the names and symbols before trying to name compounds.
Problems It is important to know the names and symbols before trying to name compounds.
Find the following elements on the periodic table and give their symbols. a. calcium b. zinc c. fluorine d. tin e. iron
Solutions
a. Calcium is a metal, so look on the left-hand side. It is in the second column, the third one down, and has the symbol Ca.
b. Zinc is a metal, so it is on the left-hand side. Looking, we find it toward the middle in the fourth row and it has the symbol Zn.
c. Fluorine in a nonmetal and is found on the right-hand side in the next-to-last column.
It has the symbol F. d. Tin is a metal, so first look on the left-hand side. Looking, we find it toward the middle right in the fifth row;it has the symbol Sn.






 Elements and compounds have names. The names vary by language but the symbol and formula of the element or compound remain the same and are controlled by an international committee (IUPAC). Some examples include magnesio in Spanish, which is magnesium in English, yet both are the symbol Mg; argento in Italian, plata in Spanish, and silver in English all have the symbol Ag; and crypto in Italian and krypton in English both have the symbol Kr. To learn chemistry, you must first know the chemistry language of the elements!
Elements and compounds have names. The names vary by language but the symbol and formula of the element or compound remain the same and are controlled by an international committee (IUPAC). Some examples include magnesio in Spanish, which is magnesium in English, yet both are the symbol Mg; argento in Italian, plata in Spanish, and silver in English all have the symbol Ag; and crypto in Italian and krypton in English both have the symbol Kr. To learn chemistry, you must first know the chemistry language of the elements! 
 Step 1. Is the element or symbol a metal? Remember to look on the left-hand side of the table. Problems It is important to know the names and symbols before trying to name compounds. Problems It is important to know the names and symbols before trying to name compounds.
Step 1. Is the element or symbol a metal? Remember to look on the left-hand side of the table. Problems It is important to know the names and symbols before trying to name compounds. Problems It is important to know the names and symbols before trying to name compounds.