
This book is dedicated to my young grandchildren, Thomas, Harper, Olive, Joanna, and Ezra, who give us all joy, wonder, and hope.
Published in 2020 by Enslow Publishing, LLC.
101 W. 23 rd Street, Suite 240, New York, NY 10011
Copyright 2020 by Enslow Publishing, LLC.
All rights reserved.
No part of this book may be reproduced by any means without the written permission of the publisher.
Library of Congress Cataloging-in-Publication Data
Names: Rybolt, Thomas R., author.
Title: Chemistry in your everyday life / Thomas R. Rybolt, PhD.
Description: New York: Enslow Publishing, 2020. | Series: Real world science | Audience: Grade 5-8. | Includes bibliographical references and index.
Identifiers: LCCN 2018049679| ISBN 9781978507654 (library bound) | ISBN 9781978509450 (pbk.)
Subjects: LCSH: ChemistryJuvenile literature.
Classification: LCC QD35 .R93 2020 | DDC 540dc23
LC record available at https://lccn.loc.gov/2018049679
Printed in the United States of America
To Our Readers: We have done our best to make sure all website addresses in this book were active and appropriate when we went to press. However, the author and the publisher have no control over and assume no liability for the material available on those websites or on any websites they may link to. Any comments or suggestions can be sent by email to .
Photo Credits: Cover, p..

Introduction
W hether you are in your home, at school, or in a store, look around you. What do you see? Lots of stuff. You may see a glass of water, bar of soap, metal coins, plastic bottles, books, or mirror with your reflection. These things are part of your everyday life.
Think about the many objects that people use every day. Plastics, fabrics, water, soap, oil, aluminum cans, soda drinks, oxygen in the air, and metal wires all have different properties and uses. Do they have anything in common? How can we understand what makes so many things that are so different? Scientists have learned something wonderful to help answer these questions. Scientists have learned that everything is made of atoms.
Chemists are the scientists who study how atoms behave and combine to make everything around us. Chemistry is the science that uses the properties and behavior of atoms to understand matter. All the stuff around you is made of atoms. So chemistry is the study of all the stuff in the world. Understanding something about atoms and ions, molecules, and chemical reactions will help you learn about the chemistry of your everyday life.
Atoms are the building blocks from which everything around you (and inside you) is made. There are 118 different types of atoms that are either found in nature or have been created by scientists in laboratories. Fewer than ninety of these types are found in nature in more than trace (very small) amounts. Many of the artificial atoms made in labs exist in only small amounts and for short times because they are radioactive.
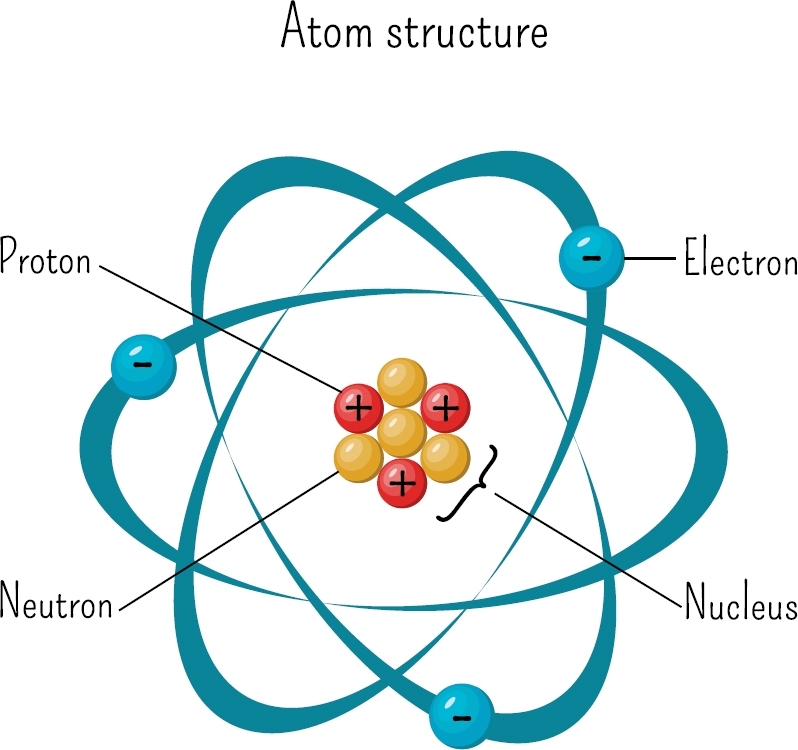
This representation of a lithium (Li) atom shows three protons and four neutrons in the nucleus and three electrons around the nucleus. The electrons would not really be in orbits but would be spread out to surround the positive nucleus like a cloud of negative charge.
Atoms are made of even smaller particles called protons, neutrons, and electrons. A proton has a positive (+) charge, a neutron has no charge, and an electron has a negative () charge. Protons and neutrons pack close together in the center of the atom, called the nucleus, which has a positive charge. The electrons in an atom form a cloud of negative charge that surrounds the positive nucleus.
A neutral atom has no charge because it has the same number of protons and electrons. If an atom loses one or more electrons, it becomes positive. If an atom gains one or more electrons, it becomes negative. Positive or negative charged atoms are called ions. Positive and negative ions are attracted to each other. They can stick together to make a solid. Table salt is called sodium chloride (NaCl). It is made of positive sodium ions (Na+) and negative chlorine ions (Cl).
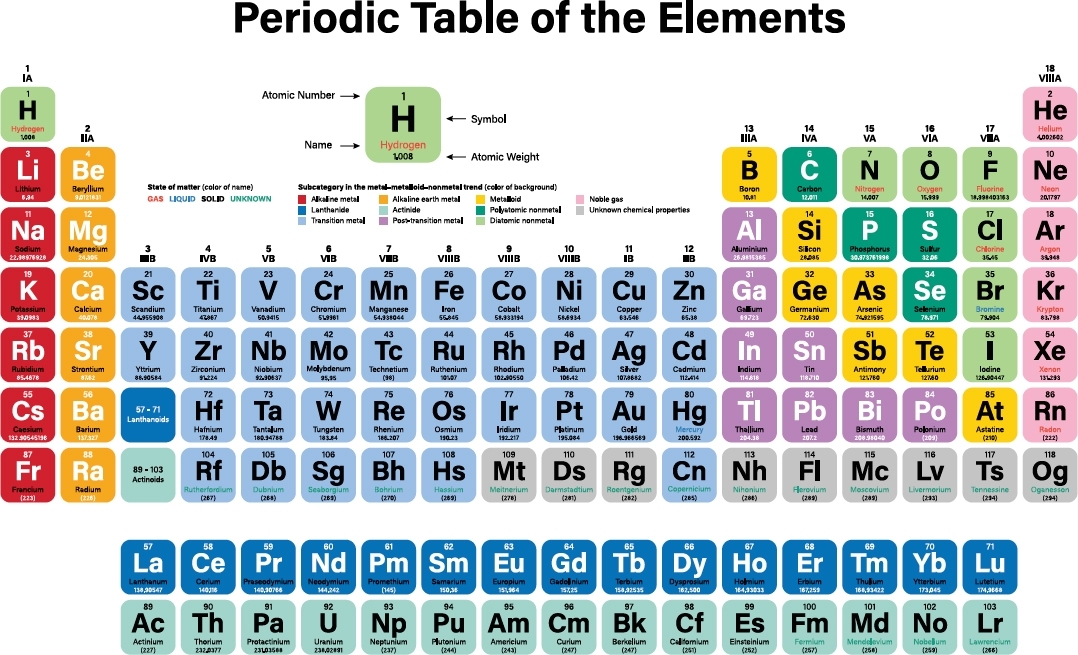
The periodic table provides a convenient way to organize the 118 known elements. It shows the element symbol, the number of protons in the nucleus, and much other useful information.
That same grain would also have an equal number of chloride ions (Cl).
Molecules are collections of atoms that are held tightly together and stay together as a group. In chemical reactions, atoms or ions can combine to make new molecules. Existing molecules can exchange atoms or combine to create new molecules. Or a larger molecule may be broken down into smaller molecules. Iron rusting, a candle burning, and bread toasting are all examples of chemical reactions. In a chemical reaction, something new is created.

Liquid of Life
Y ou drink it. You wash with it. You see it near the ground as fog. You see it in the sky as clouds. It falls from the sky as rain or snow. It is essential for life. What is it? Water.
Molecules at a Glance
The building block of water is the molecule H2O. Atoms next to each other share a pair of electrons between them to form a chemical bond. The chemical bonds throughout the molecule hold the atoms together. Some molecules are found in nature. Other molecules are made by chemists who put atoms together in new ways. Millions of different kinds of molecules have been identified and studied! New molecules are being discovered and made every day.
Some molecules have many thousands of atoms, while others have just a few atoms. For example, carbon dioxide gas found in the air is written as CO2. Each molecule of CO2 has one carbon atom (C) and two oxygen (O) atoms. Table sugar is a molecule called sucrose, and its formula is C12H22O11. Sucrose has twelve carbon (C) atoms, twenty-two hydrogen (H) atoms, and eleven oxygen (O) atoms held tightly together.
Molecules of Water
H2O is made up of one oxygen (O) atom and two hydrogen (H) atoms held together by electron sharing to make two chemical bonds. The electron clouds around the atoms are negative, and the nuclei at the center of the three atoms are positive. This Water molecules are extremely small because they are made of only three atoms. To fill a one-liter bottle takes about 34,000,000,000,000,000,000,000,000 (thirty-four septillion) water molecules!
The tiny molecules of H2O have amazing properties. None of us could live without water. Most small molecules of only a few atoms are gases, not liquids, at what scientists call room temperature (77oF or 25oC). For example, carbon dioxide (CO2) and sulfur dioxide (SO2) are gases at ordinary temperatures. Why is water (H2O) a liquid?
Each water molecule has a slightly positive side where the two hydrogen atoms are located. It also has a slightly negative side where electrons are more concentrated. This distribution of positive and negative charges allows each water molecule to have a special attraction to up to four other water molecules. The slightly positive H atom on one water molecule can be attracted to the slightly negative O atom on a different water molecule. This attraction is called hydrogen bonding. Hydrogen bonding helps hold the water molecules together despite their small size.
Next page


















