Page List

For our future scientists
Published in 2009 by The Rosen Publishing Group, Inc.
29 East 21st Street, New York, NY 10010
Copyright 2009 by The Rosen Publishing Group, Inc.
First Edition
All rights reserved. No part of this book may be reproduced in any form without permission in writing from the publisher, except by a reviewer.
Library of Congress Cataloging-in-Publication Data
Lew, Kristi.
Mercury / Kristi Lew.1st ed.
p. cm.(Understanding the elements of the periodic table)
Includes bibliographical references and index.
ISBN-13: 978-1-4042-1780-5 (library binding)
1. Mercury. 2. Periodic lawTables. 3. Chemical elements. I. Title.
QD181.H6L49 2009
546.663dc22
2007044919
Manufactured in the United States of America
On the cover: Mercurys square on the periodic table of elements. Inset: The atomic structure of mercury.
Contents
S ometimes, doctors have to be detectives, too. For example, in 1989, a family in Michigan visited their doctor when one of the sons came down with a mysterious illness. The skin on the boys hands had turned scaly and pink. He was drooling, angered easily, and was hard to manage. After many examinations and medical tests, the boys doctors discovered that he was suffering from pink disease. Pink disease is considered rare today, but at one time, it was a common medical problem in the United States.
The skin on the hands and feet of people who suffer from pink disease often peels off, giving these extremities a pink color. This discoloration of the hands and feet gives the disease its name. The scientific name for pink disease is acrodynia. The illness is caused when babies or young children are exposed to the element mercury (chemical symbol: Hg). In the past, mercury was an ingredient in some teething powders. These powders were used to soothe sore gums caused by emerging teeth. Unfortunately, the element mercury is also poisonous. In 1947, when doctors discovered that mercury was the cause of pink disease, the element was removed from teething powders and the disease became quite rare.
By the 1990s, after that Michigan family arrived at the doctors office, the disease was not often seen in patients. It turned out that the familys home had recently been repainted. The house paint contained a chemical additive called phenylmercury(II) acetate (C8H8HgO2). At the time, this form of mercury was not known to cause humans any harm. It was added to the paint to prevent the growth of mildew. Some of the mercury in the paint vaporized, or turned into a gas. This gas is colorless and odorless. The entire family breathed in some of the mercury vapor and suffered from varying degrees of mercury poisoning. Inhaled mercury vapor can be very dangerous.
Mercury is a cumulative poison, meaning that it builds up in the body over time. It is also a neurotoxin. Neurotoxins can destroy nerve tissue, including the brain. Chronic, or long-term, mercury exposure can lead to tremors (uncontrollable shaking or trembling), extreme mood changes, hearing loss, and blindness. These factors make exposure to even small amounts of mercury extremely dangerous. Mercury can enter the body through the skin, its vapor can be breathed in through the lungs, or it can enter through the digestive tract if food contaminated with mercury is eaten.

Workers in hat factories were often exposed to dangerous mercury vapors. Mercury, a neurotoxin, can build up in the body and destroy nervous tissue, including brain tissue.
The saying mad as a hatter is also thought to have come from the use of a mercury compound, called mercury(II) nitrate, in the hat-making trade. This compound was used to make felt out of rabbit fur, and hats were manufactured with the felt. Workers in hat factories often suffered from serious health problems, such as the loss of teeth and hair, memory loss, and the deterioration of their nervous systems. This general breakdown of nervous tissue, especially in the brain, may have caused mental derangement and bizarre behavior, causing people to coin the term mad as a hatter. Because of the health risk of mercury exposure, the U.S. Public Health Service banned the use of mercury(II) nitrate in the felt industry on December 1, 1941.
M ercury is the only metal that is a liquid at room temperature (68 Fahrenheit [20 Celsius]). Only one other element, the nonmetallic element bromine (Br), is a liquid under these conditions. Elements are substances that cannot be broken down into something simpler by using ordinary chemical means, such as exposing them to acids, electricity, or heat.
The History of Mercury
Mercury is a naturally occurring element in Earths crust. It was known to the ancient Greeks, Romans, Chinese, and Hindus. Samples of the metal have been found in 3,500-year-old Egyptian tombs.

Mercury is a liquid at room temperature. Due to its appearance and how it moves, its also called quicksilver.
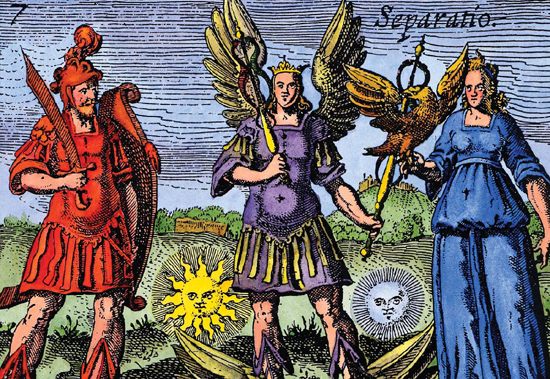
Because of its mobility, mercury was named for the Roman god Mercury (center), the swift messenger of the gods. Alchemists believed mercury, sulfur, and salt were the three main substances that made up Earth.
Mercury was also a metal known to alchemists during the Middle Ages. The alchemists considered mercury, sulfur (S), and salt to be the three main substances that made up Earth. In fact, the Hindi word for alchemy, rasasiddhi, means knowledge of mercury. Alchemists believed that all metals were mixtures of mercury and other substances. At the time, alchemists knew of seven metals: mercury, gold (Au), silver (Ag), copper (Cu), tin (Sn), lead (Pb), and iron (Fe). They also believed that, given the right combination of ingredients, mercury could be transmuted, or changed, into gold. Unfortunately for the alchemists, one element cannot be changed into another element through ordinary chemical means. So, no matter how hard they tried, they were unsuccessful at turning mercury, or any other metal, into gold.
Mercurys toxic effects were also known to the ancients. They could see the illnesses suffered by miners who dug mercury compounds from the ground. These ailments started with tremors and progressed to full-blown mental derangement, or madness.
Mercurys chemical symbol is Hg. The Hg comes from the Greek word hydrargyrum. Hydrargyrum means liquid silver. Because of its silvery appearance and the way it moves, the metal was also called quicksilver. In fact, the element is named for the Roman god Mercury, who was known for his speed and mobility.

Archaeologists studied these cinnabar-covered remains in the ruins of Copan, Honduras. Ancient Mayans sometimes painted the remains of their dead with cinnabar, a bright red mineral of mercury.
Where Is Mercury Found?




















