
Editorial
Rob Franek, VP Test Prep Books, Publisher
Seamus Mullarkey, Associate Publisher
Laura Braswell, Senior Editor
Selena Coppock, Editor
Heather Brady, Editor
Random House Publishing Group
Tom Russell, Publisher
Nicole Benhabib, Publishing Manager
Ellen L. Reed, Production Manager
Alison Stoltzfus, Managing Editor
The Princeton Review, Inc.
111 Speen Street, Suite 550
Framingham, MA 01701
E-mail:
Copyright 2011 by The Princeton Review, Inc.
The Princeton Review is not affiliated with Princeton University
All rights reserved. Published in the United States by Random House, Inc., New York, and in Canada by Random House of Canada Limited, Toronto.
eISBN: 978-0-307-94436-8
ISSN: 1092-0102
Editor: Anya Yurchyshyn
Production Editor: Stephanie Tantum
Production Coordinator: Deborah A. Silvestrini
2012 Edition
v3.1
ACKNOWLEDGMENTS
Id like to thank John Katzman for entrusting me with this project and also my editor, Rebecca Lessem. For her work on past editions, Id like to thank Rachel Warren, whose guidance and tireless effort helped me to convert chemistry into English. Id also like to thank Eric Payne, whose rigorous attention to detail helped me to separate chemistry from fantasy. Id like to thank Tom Meltzer for his advice and Libby OConnor for her patience.
Id also like to thank The Princeton Reviews editorial and production crew. Thanks also to Robbie Korin, James Karb, and Chris Volpe for their suggestions and corrections.
CONTENTS
Introduction
WHAT IS THE PRINCETON REVIEW?
The Princeton Review is an international test-preparation company with branches in all major U.S. cities and several abroad. In 1981, John Katzman started teaching an SAT prep course in his parents living room. Within five years, The Princeton Review had become the largest SAT prep program in the country.
Our phenomenal success in improving students scores on standardized tests is due to a simple, innovative, and radically effective philosophy: Study the test, not just what the test claims to test. This approach has led to the development of techniques for taking standardized tests based on the principles the test writers themselves use to write the tests.
The Princeton Review has found that its methods work not just for cracking the SAT, but for any standardized test. Weve already successfully applied our system to the GMAT, LSAT, MCAT, and GRE, to name just a few. Obviously, you need to be well versed in chemistry to do well on the AP Chemistry Exam, but you should remember that any standardized test is partly a measure of your ability to think like the people who write standardized tests. This book will help you brush up on your AP Chemistry and prepare for the exam using our time-tested principle: Crack the system based on how the test is created.
We also offer books and online services that cover an enormous variety of education and career-related topics. If youre interested, check out our website at PrincetonReview.com.
1
Orientation
WHAT IS THE AP PROGRAM?
The Advanced Placement (AP) Program bridges high schools and colleges by allowing high school students to do college-level work for college credit. The AP Chemistry Exam is one of more than 30 college-level examinations offered every year.
AP courses are offered by more than 10,000 high schools in the United States, Canada, and more than 60 additional countries. More than 3,000 colleges around the world offer college credit to students who perform well on AP tests. The specific score required for credit varies from school to school and from subject to subject.
The AP Program is coordinated by the College Board. The College Board is a national nonprofit organization composed of representatives from various schools and colleges. They see it as their mission to set educational standards.
The College Board appoints a development committee for each of the subjects. The development committee decides what should be covered in an AP course and how it should be covered on the AP test. The AP Chemistry development committee is composed of three high school chemistry teachers, three college professors who teach general chemistry, and an additional college professor who chairs the group. Each member of the development committee serves a three-year term.
The test is administered by the Educational Testing Service (ETS)the same folks who bring you the SAT. ETS also plays a role in developing the test.
WHAT IS THE AP CHEMISTRY EXAM?
The AP Chemistry Exam is a three-hour-long, two-section test that attempts to cover the material you would learn in a college first-year chemistry course. The first part, which counts for 50 percent of your grade, consists of multiple-choice questions. The second part, which counts for 50 percent of your grade, is composed of free-response questions, such as short essays and problems involving calculations.
The test is offered once every year in May. Its scored in June. The multiple-choice section is scored by computer and the problems and essays are scored by a committee of high school and college teachers. The problems and essays are graded according to a standard set at the beginning of the grading period by the chief faculty consultants. Inevitably, the grading of Section II is never as consistent or accurate as the grading of Section I.
When the grading is done, the results are curved and each student receives a grade based on a five-point scale. For the AP Chemistry Exam, the results break down as follows:
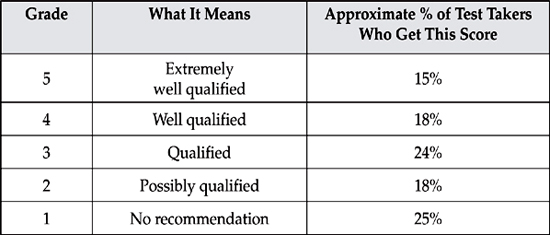
Although standards vary from school to school, its safe to say that most colleges will give credit for a 5, some will give credit for a 4 or 3, and very few will give credit for a 2.
CRACKING THE MULTIPLE-CHOICE SECTION
T HE B ASICS
Section I of the test is composed of 75 multiple-choice questions, for which you are allotted 90 minutes. This part is worth 50 percent of your total score.
For this section, you will be given a periodic table of the elements and you may NOT use a calculator. The College Board says that this is because the new scientific calculators not only program and graph but also store informationand they are afraid youll use this function to cheat!
The first 15 multiple-choice questions, give or take a few, will be formatted with 5 answer choices followed by a series of questions (as shown on the next page).
Questions 14
(A)O2
(B)H2O
(C)Ni
(D)Fe
(E)NaCl
1. This species contains ionic bonds. (E)
2. This species is a gas at standard temperature and pressure. (A)
3. This species is denser as a liquid than as a solid. (B)
4. This species contains a double bond. (A)
These are mostly straightforward, you know it or you dont questions. Notice that an answer can be used once, more than once, or not at all.
The rest of the multiple-choice questions are in the standard question-and-answers format shown below.







