1. Introduction
There are several disorders which carry an increased risk of thrombosis, clots that interfere with normal circulation, including: venous thromboembolism (VTE), comprising both deep vein thrombosis (DVT) and pulmonary embolism (PE), atrial fibrillation (AF), acute coronary syndromes (ACS), valve disease and endocarditis, and conditions associated with a raised risk of ischemic stroke. Due to this increased risk, a number of thromboprophylactic medications target the coagulation cascade. Effective anticoagulation can be achieved by inhibiting different coagulation factors in the coagulation cascade.
1.1 Overview of the Coagulation Cascade
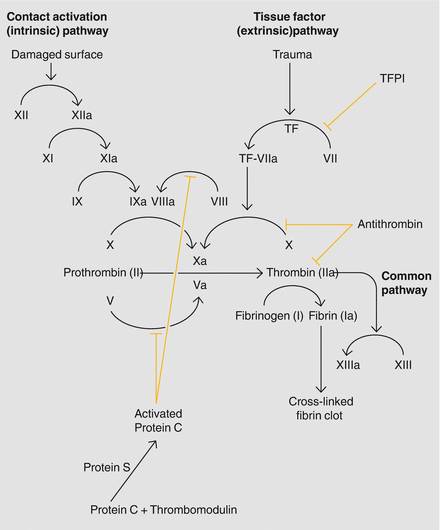
The coagulation cascade is composed of two pathways; the intrinsic, or contact activation pathway and the extrinsic, or tissue factor pathway (Fig. ]. If a hemorrhage occurs due to blood vessel injury, the coagulation system facilitates hemostasis.
Figure 1.1
The coagulation cascade
A series of reactions occur throughout the coagulation cascade causing the conversion of inactive enzyme precursors to their active forms; these active forms catalyze the subsequent reactions in the cascade. The coagulation cascade prevents both excessive bleeding and excessive coagulation through a negative feedback loop, self-maintained by a regulated balance of procoagulant and anticoagulant factors.
In the extrinsic (tissue factor) pathway [] when the coagulation cascade is activated tissue factor (TF) is released from subendothelial tissue and comes into contact with factor VII which, in the presence of Ca2+, forms the activated complex TF-VIIa. TF-VIIa catalyzes the conversion of factor X to factor Xa which, along with activated factor V, initiates the formation of the serum protease thrombin, formed when factors Va and Xa cleave prothrombin fragments 1 and 2. Thrombin then cleaves fibrinopeptides A and B from fibrinogen resulting in insoluble fibrin.
In the intrinsic (contact activation) pathway [] damage to the endothelial surface causes the formation of factor XIIa from factor XII, which in turn triggers formation of factor XIa from factor XI. Factor XIa catalyzes the conversion of factor IX to factor IXa (in the presence of Ca2+), which triggers the conversion of factor VIII to factor VIIIa. Factors IXa and VIIIa form a catalytic complex and activate factor X. The formation of factor Xa marks the convergence of the extrinsic and intrinsic pathways into a common pathway, which is responsible for the formation of a fibrin mesh on the damaged vessel wall.
1.2 Current Approaches to Anticoagulation
Currently vitamin K antagonists (VKAs), such as warfarin, are the mainstay of anticoagulant treatment. VKAs have been the oral anticoagulant of choice for more than 50 years, with warfarin being the most commonly used VKA worldwide due to its low cost and once-daily (qd) dosing [].
Warfarin is highly effective in the prevention of stroke and recurrent VTE, showing improved efficacy over antiplatelet therapy [].
Warfarin inhibits the normal production of several anticoagulation factors that are dependent upon vitamin K []. When vitamin K is available these anticoagulation factors are -carboxylated leading to the expression of their procoagulation activity. The availability of vitamin K depends on the normal functioning of the enzyme vitamin K epoxide reductase. Warfarin inhibits this enzyme leading to a lack of vitamin K resulting in the production of functionally impaired, partially carboxylated and decarboxylated factors with corresponding anticoagulant effects.
Despite their efficacy, there are limitations associated with VKA treatment including: a number of drug and food interactions, individual dose-adjustment based on international normalization ratio (INR), maintenance of INR within the time in therapeutic range (TTR), and the need for constant monitoring.
The number of food and drug interactions associated with VKA use results in a substantial variability in the absorption and bioavailability of warfarin []. Additionally, the consumption of alcohol can interact with liver enzymes which have the potential to affect the metabolism of warfarin. Furthermore, it often takes several days to reach therapeutic levels due to its long half-life (approx. 40 h). Warfarin is mainly bound to albumin in the plasma and metabolized in the liver.
The effectiveness and safety of warfarin are critically dependent on maintaining the INR within a therapeutic range of 2.03.0. Due to the wide number of pharmacological interactions, warfarin requires regular monitoring of its anticoagulant activity and patients require individual dose adjustment.
Treatment with warfarin is associated with a number of side-effects, including increased risk of severe hemorrhagic complications, such as intracranial hemorrhage. Over-anticoagulation (INR >3.0) carries a high risk of intracranial hemorrhage, which accounts for ~90 % of deaths from warfarin-associated hemorrhage [].
1.3 The Ideal Anticoagulant
The limitations described above complicate the use of these drugs, especially in the long term setting, and there remains a need for anticoagulants that are at least as effective but safer and more convenient. The development of new anticoagulants has identified a number of properties for an ideal anticoagulant.
The ideal anticoagulant should have a quick onset of action that remains stable throughout the day. Additionally, it should target specific coagulation factors within the coagulation cascade, preferably acting independently of cofactors, with predictable anticoagulant effects, and fixed doses. Ideally, only pathological thrombosis leading to thromboembolic events would be inhibited, but a certain extension of activity of the coagulation system that maintains physiological hemostasis should be allowed [].
From a clinical perspective, the ideal anticoagulant should have easy oral application, a wide therapeutic window, predictable pharmacodynamic and pharmacokinetic properties, and a low risk for interactions with other drugs or food, allowing for a simple dosing regimen without the need for regular monitoring. Moreover, it would have a reversible anticoagulant effect for emergency situations and the risk of hemorrhage and effects on other organs should be minimal.
A better understanding of the coagulation cascade led to identification of many targets for novel anticoagulants in the coagulation pathway. Indeed, a number of non-VKA oral anticoagulants (NOACs) have been launched or are currently in development, which have the potential to overcome the limitations associated with VKAs. With the use of NOACs, such as direct oral thrombin inhibitors (dabigatran) or factor Xa inhibitors (apixaban, rivaroxaban, edoxaban), some of the inherent problems such as regular INR monitoring, dose adjustment, and food and drug interactions, will be removed.
OpenAccess This chapter is distributed under the terms of the Creative Commons Attribution Noncommercial License, which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
Mackman N. Triggers, targets and treatments for thrombosis. Nature. 2008;451:9148. CrossRef PubMedCentral PubMed