Springer-Verlag Berlin Heidelberg 2015
Thomas Bieber and Frank Nestle (eds.) Personalized Treatment Options in Dermatology 10.1007/978-3-662-45840-2_1
1. Concept and Scientific Background of Personalized Medicine
1.1 Introduction
In the history of medicine, it has always been the goal to understand the basic mechanisms leading to diseases and to develop appropriate therapeutic approaches based on this knowledge. Over the last several centuries, in the absence of appropriate pathophysiological knowledge, the development of medical care has been dominated by a rather empirical approach. In the end of the last century, the need for more scientific and economic evidences in the wide choice for appropriate treatment regimen became a primary goal. However, this approach was not able to take into account the wide heterogeneity of almost all diseases and the pharmacological development was driven by the idea of generating possibly one or a few medical products aimed to treat a large population of patients affected by one given disease. This kind of one size fits for all approach was of benefit for some selected situations such as pain or headache, while it became obvious that diseases such as various kinds of cancer were hardly responding to this classical approach [].
The idea of personalized medicine can also be found in the literature under more or less synonymous terms [).
Table 1.1
Key fields and potential of personalized medicine in dermatology
Key fields |
Heterogeneity of a given target disease |
Identification and validation of biomarkers and their development as companion diagnostic |
Stratification of patient population with the biomarker/endophenotype |
Improved genotype-phenotype relationship with information of improved computational medicine |
Provide evidence for a better benefit-to-risk ratio and efficiency |
Potential of personalized medicine in dermatology |
Identification of still healthy individuals with high risk to develop a given disease and the opportunity to act preventively (e.g., atopic dermatitis) |
Opportunity for early detection of a disease possibly even before the first symptoms appear (early intervention) and to control them effectively (e.g., psoriasis arthritis) |
Better and more precise diagnostic of disease and stratification according to ways for a more adapted therapy (e.g., malignant melanoma) |
Prognostic information (e.g., autoinflammatory skin diseases, skin cancers) |
Development of more targeted therapies with more efficacies and less side effects (e.g., lupus, malignant melanoma) |
Reduce the time, costs, and failure rate of clinical trials for new therapies |
Stage adapted therapy decisions and improved treatment algorithms (e.g., skin cancers) |
Better monitoring during therapy and more options for alternatives by nonresponders (e.g., skin cancers) |
Opportunity for disease-modifying strategy (e.g., skin cancers, atopic dermatitis) |
1.2 The Concept and Goals of Personalized Medicine: The Right Patient with the Right Drug at the Right Dose at the Right Time
With the elucidation of the human genome at the beginning of this century [] and avoiding to expose unresponsive patients to the same drug with potential side effects will overall increase the effectiveness of a given medial product and decrease the risk for the generation of unnecessary side effects or drug interactions which may induce severe complications and costs.
1.3 The Tools of Personalized Medicine
The biomarkers are the most important tools on which personalized medicine strategies will be based on in the future [], will be of crucial importance to be included in the strategies mentioned herein. Therefore, establishing and combining (1) high-quality biobanks gathering representative biological samples, (2) high-quality phenotypic information, and (3) state of the art in systems biological tools are considered to be key for the discovery and validation of biomarkers.
1.4 Dissecting the Complex Clinical Phenotypes for Optimized Drug Development and Application
Each disease is characterized by a more or less wide spectrum of individual symptoms building up a complex clinical phenotype but under the heading of one diagnosis. This clinical heterogeneity often mirrors complex pathophysiological mechanisms which may have distinct epi/genetic origins. Similarly, the heterogeneity of the clinical response to the classical treatments includes the risk to apply potent drugs with serious side effects in patients who will not respond to that particular drug [] which cannot be addressed in this short review but are of real concern at all levels.
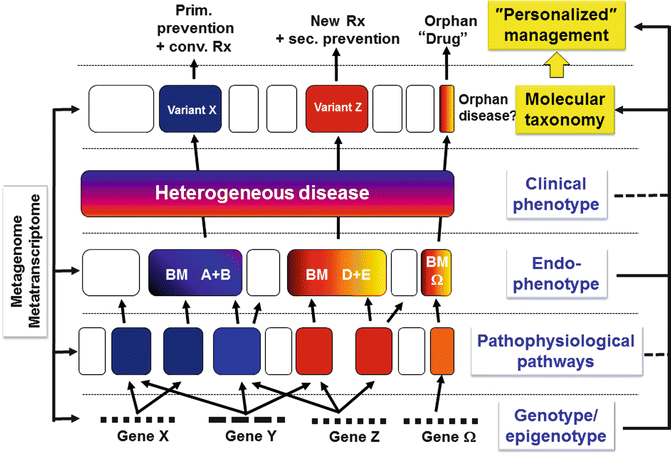
Fig. 1.1
Endophenotype-based stratification of heterogeneous clinical phenotypes into variants and the consequences for personalized management
1.5 Conclusion and Outlook
As a consequence of tremendous progress in biomedical research and diagnostic technologies, an endophenotype-based stratification of complex clinical phenotypes will allow to better address the patient population which will have the highest benefit of targeted therapy with a significantly improved safety profile. The combination of several biomarkers with different predictive and prognostic values [].
While personalized medicine has experienced its innovative start in the field of life-threatening diseases with significant unmet medical needs, such as oncology and neurological diseases, it is expected that this trend will extend progressively to other fields such as autoinflammatory and autoimmune diseases. The further reduction of the costs for sequencing and overall genomics-based diagnostics will lead to its implementation to more and more fields less related to the unmet medical need but rather to, e.g., aging-related issue and ultimately to lifestyle aspects [].
The wider acceptance and application of validated and qualified genomic markers may initiate a new medical evolutionary process, progressively shifting away from the traditional curative medicine. This putative future health system involves a transition to predictive, preventive, personalized, and participatory (P4) [] medicine and will require a systems biologic approach including the collection of tremendous amounts of data from genomics, endophenotypic information, as well as those related to individual interactions with the environment. However, legal and ethical considerations in the context of an increasing risk of transparency should guarantee the privacy and autonomy of choice and decision of all individuals and patients. Otherwise, an uncontrolled overemphasizing of the significance of individual genomic information could lead the society into temptation to decide on an obligation of prevention for each individual.