Part I
Neurophysiology
Springer-Verlag London 2016
Laurent Misery and Sonja Stnder (eds.) Pruritus 10.1007/978-3-319-33142-3_1
1. Neural Processing of Itch
Tasuku Akiyama 1
(1)
Departments of Dermatology & Anatomy and Cell Biology, Temple Itch Center, Temple University, 3500 N Broad Street MERB452, Philadelphia, PA 19140, USA
(2)
Department of Neurobiology, Physiology & Behavior, University of California, Davis, 1 Shields Avenue, Davis, CA 95616, USA
Tasuku Akiyama (Corresponding author)
Email:
E. Carstens (Corresponding author)
Email:
Keywords
Itch Pruritus Sodium channels TRP channels Spinal cord Pruriceptors Glia Mrgprs GRP NPPB PAG
Introduction
Itch is a unique somatosensation arising from the skin and mucous membrane, but not internal organs. There are many different types of itch mediators including endogenous pruritogens released from neuronal as well as non-neuronal cells, such as keratinocytes and immune cells, as well as exogenous pruritogens from the external environment (See Chap. ). These itch mediators activate their cognate receptors expressed by nerve endings of primary sensory neurons. The first part of this chapter will describe the molecular mechanisms of itch transduction by primary sensory neurons.
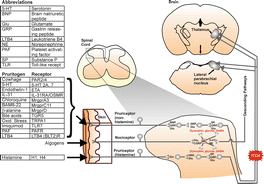
Primary sensory afferents convey itch information to secondary sensory neurons located in the spinal and trigeminal dorsal horn (Fig. ). The second part of this chapter will describe recent findings regarding the spinal processing of itch signals.
Fig. 1.1
Schematic drawing of neural pathway for itch. Tables to left show abbreviations ( upper ), non-histaminergic itch mediators and their receptors ( middle ), and histamine and its receptors ( lower ). Drawing to right shows itch pathway from cutaneous pruriceptors to spinal cord to higher levels of the brain. Red minus sign () shows inhibitory interneuron. Neuropeptides and transmitters shown in black text are excitatory, while those in red are inhibitory
Primary Afferents
Type of Nerve Fibers
Itch stimuli impacting the skin are transmitted through primary sensory neurons, whose peripheral terminals are located in the epidermis. Their central branch projects to the spinal cord or medullary dorsal horn where the cell bodies and dendrites of second-order sensory neurons are located. The primary sensory afferents are categorized into three classes; A-, A- and C-fibers, based on their diameters and conduction velocities. The A-fibers having a nerve conduction velocity of above 30 m/s are thickly myelinated fibers that relay information of light touch and pressure. The thinly myelinated A-fibers and the unmyelinated C-fibers have a nerve conduction velocity of around 230 m/s and less than 2 m/s, respectively, and mediate the sensations of itch as well as pain. Histamine, and cowhage whose spicules contain pruritogenic proteins, apparently activate different subpopulation of C-fibers and A-fibers. Mechono-insensitive C-fibers preferentially respond to histamine but not cowhage [].
Mrgprs
The family of Mas-related G protein-coupled receptors (Mrgprs) comprises 18 genes in humans and 50 genes in mice. They are exclusively expressed in the primary sensory neurons and mast cells. Certain Mrgprs (i.e., MrgprA3, MrgprC11, and MrgprD) have been shown to mediate itch [].
TRP Channels
TRP channels participate in the transduction of thermal, osmotic, and chemosensory stimuli including pruritogens. TRPV1 and TRPA1 channels play a crucial role in pain. They are apparently activated downstream of GPCRs in itch transduction. Binding of histamine to the H1 receptor which is a Gq/11 type GPCR causes activation of TRPV1 possibly via 12-HETE, an arachidonic acid cascade metabolite []. Multiple TRP channels plausibly are involved in transduction of itch evoked by a single pruritogen.
TRPV3 is activated by warm temperatures (range 3339 C) and is predominantly expressed in keratinocytes []. TRPV3 thus plausibly has a role in the development of chronic itch.
TRPV4 is another TRP channels activated by moderately warm temperatures (range 2734 C) and is expressed in the sensory neurons as well as the keratinocytes in the skin. TRPV4 mRNA was upregulated in the skin with itching burn scars [].
Sodium Channels
The voltage-gated sodium channels play a crucial role in the generation of action potentials in sensory neurons. There are nine isoforms of the voltage-gated sodium channel (i.e., Nav1.11.9). It has been reported that Nav1.3, Nav1.7, Nav1.8, and Nav1.9 are expressed in DRG neurons and implicated in pain []. Nav1.8 may be activated downstream of MrgprA3 and TRPA1.
Certain sea creatures produce toxins that target sodium channels, such as tetrodotoxin from puffer fish and muO-conotoxinMrVIB from Conusmarmoreus []. A sodium channel that is targeted by ATX- might play a unique role in itch transmission (e.g., A-fiber-mediated itch).
Spinal Cord
Neurotransmitters
Various neuropeptides as well as glutamate mediate the transmission of itch from the central terminal of primary sensory afferents to the secondary sensory neurons in the spinal cord. Gastrin-releasing peptide (GRP) [].
Projection Neurons
Spinal neurons have been categorized by different criteria (e.g. morphology, bursting pattern, and molecular markers). Certain molecular markers apparently classify spinal neurons into functionally distinct population. Dorsal horn neurons that project to thalamus or parabrachial nuclei in rodents express the neurokinin1 (NK1) receptor [). To date, however, there are no studies of itch-related neural activity in cortical circuits activated by ascending spinothalamic and spinoparabrachial pathways.
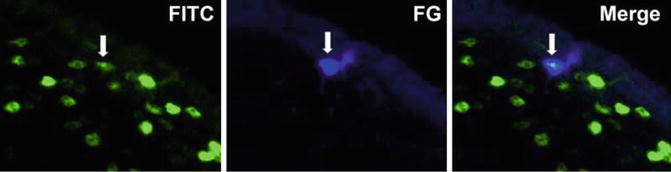
Fig. 1.2
Example of double-labeled neuron. Left panel shows Fos-immunoreactive neurons (FITC). Middle panel shows one neuron labeled with FG (Fluorogold; retrograde tracer). Right panel shows merged images, with the double-labeled neurons exhibiting a teal hue (With permissions from John Wiley and Sons, [)
Excitatory Interneurons
Excitatory interneurons play a significant role in the spinal transmission of pain and/or itch. Ablation of somatostatin-expressing excitatory neurons reduced mechanical pain without affecting the senses of innocuous touch, heat or cold [].
Inhibitory Interneurons
Inhibitory interneurons can be classified into four groups according to the expression of galanin, neuronal nitric oxide synthase (nNOS), neuropeptide Y and parvalbumin [].
Glial Cells
Recent studies have provided evidence for a critical role of spinal glial cells in chronic pain. They release pro-nociceptive mediators such as ATP, cytokines and chemokines to initiate and maintain chronic pain. The precise role of glial cells in itch is still unknown, but there are several reports supporting their role in itch []. Scratching and/or skin inflammation apparently causes astrogliosis through the activation of the STAT3 pathway in the astrocytes.