Springer-Verlag Berlin Heidelberg 2012
Efstathios E. (Stathis) Michaelides Alternative Energy Sources Green Energy and Technology 10.1007/978-3-642-20951-2_1
1. Energy Demand and Supply
Efstathios E. (Stathis) Michaelides 1
(1)
Department of Engineering, TCU, Fort Worth, TX, USA
Efstathios E. (Stathis) Michaelides
Email:
Abstract
The use of energy defines the beginning of human civilization: when the prehistoric human mastered the use of fire for domestic comfort and cooking, human civilization began and evolved to reach the age of the locomotive, the nuclear power plant, the automobile, the airplane, the personal computer and the wireless internet. Throughout the centuries, the human society has evolved by increasingly using energy to the point where the consumption of energy is necessary for the functioning of the contemporary society, the prosperity of the nations and the survival of our civilization. Energy is produced and is being used in different forms: airplanes and automobiles use liquid hydrocarbon fuels; electric power plants convert primarily the energy in coal, natural gas, nuclear and hydroelectric into electricity; and a contemporary household uses electricity and natural gas for domestic comfort, entertainment and the preparation of meals. Because most functions of our society are based on the use of energy, elaborate networks of energy supply have been developed in the last three centuries: electricity is fed into communities by the transmission lines of the electric grid at high voltage; natural gas by a complex system of pipelines, which transcend national boundaries; and tanker ships crisscross the oceans daily to supply crude oil to refineries. The economic impact of the energy supply and the energy trade is of paramount importance to all nations. The geopolitical activities of most modern nations are significantly influenced by their need for a constant and secure energy supply. Most of modern wars (those after 1950) have been fought for the control and security of energy supplies and many treaties and international agreements have been cemented with energy resources as the primary issue. This is a general chapter on energy, not just alternative energy, which explains in a quantitative way what is the quantity we call energy, whence it comes and where it goes. The forms that energy is produced and consumed are, first, explained. The several units that are commonly used for different energy forms and quantities are listed and their equivalencies are explained. Secondly, the importance of energy in the economic activities of the contemporary society is described qualitatively and the primary energy resources are identified. The current energy trade between groups of nations is also described briefly and the main flow of primary energy resources is identified. Thirdly, historical data on energy production and consumption in several groups of nations are offered as well as some acceptable predictions for the future demand and supply of energy.
1.1 Forms and Units of Work, Heat and Energy
From the theory of Newtonian Mechanics, work has been defined as the scalar product of a force and the distance its point of application moves during a period of time. Power is the work per unit time. In the case of a force that is variable, the work is given by an integral, which describes the motion of the force between two end points, a and b . The work and its time derivative, the instantaneous power, are defined as follows:
Power is the scalar product of the force and of the velocity its point of application moves. For an engine to produce or consume power there must be forceful motion of some of its components. Usually, this motion is circular and is converted by gears to linear motion.
All the engines that are currently used for the performance of the several tasks desired by the human society produce work and consume energy resources. For example, the internal combustion engine consumes gasoline or diesel and produces work, which is used in propulsion. Energy is a very broad concept and is defined as the potential of materials and systems to perform useful work. Energy is measured in the same units as work and comes in several forms. Heat or thermal energy is a form of energy that is transferred from materials at higher temperatures to materials at lower temperatures. It is usually produced by the combustion of fuels, which are regarded as energy sources. There are several engineering systems, such as the power plants and the internal combustion engines, which convert heat into work. Other forms of energy, which are commonly met in engineering practice and everyday applications, are:
The potential energy ( mgz ) of matter, at a higher level.
The kinetic energy (1 / 2 m
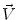
) of matter that moves with velocity
.
The chemical energy ( G ) of substances, such as coal and hydrocarbons.
The elastic energy ( Vm ) of a stressed solid with volume V and strain .
The electric energy ( qV ) of a charge q in a voltage difference V .
The magnetic energy ( HM ) of a magnetic quantity M in a magnetic field with intensity H .
The wave energy (1/2 Ag ) of waves on an area A with amplitude .
The nuclear energy ( mc ) which is equivalent to the mass, m .
The surface energy ( A ) of fluids with surface tension and area A .
Our society uses the energy content of several substances to produce work and to accomplish several tasks and activities. Different forms of energy undergo conversions and produce work during these activities. The energy conversions are subjected to the Laws of Thermodynamics. Details of these laws, a general overview of the subject of Thermodynamics, and examples on the conversions of energy forms are given in .
1.1.1 Units of Energy
From the kinetic energy of a single electron that may have been created during the electronhole pair process in a photovoltaic cell, to the net power produced in a large nuclear power station, the units of energy span a very large range. Although they are different concepts and are not interchangeable, work, heat and energy are measured by the same units. The unit of energy in the Systeme Internationale (S.I.) is the Joule (J) and is defined as the work done when a constant force of 1 Newton (N) moves its point of application by 1 meter (m). One Joule is also performed when a charge of 1 Coulomb (1 Cb) moves through an electric potential difference of 1 Volt (1 V). Multiples of the Jouleas well as of all the other S.I. unitshave been defined by international convention and are as follows:
1 yocto-Joule (yJ) = 1024 J
1 zepto-Joule (zJ) = 1021 J
1 atto-Joule (aJ) = 1018 J
1 femto-Joule (fJ) = 1015 J.
1 pico-Joule (pJ) = 1012 J.
1 nano-Joule (nJ) = 109 J.
1 micro-Joule (J) = 106 J.
1 milli-Joule (mJ) = 103 J.
1 kilo-Joule (kJ) = 103 J.
1 mega-Joule (MJ) = 106 J.
1 giga-Joule (GJ) = 109 J.
1 tera-Joule (TJ) = 1012 J.
1 peta-Joule (PJ) = 1015 J.
1 exa-Joule (EJ) = 1018 J
1 zetta-Joule (ZJ) = 1021 J
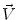 ) of matter that moves with velocity
) of matter that moves with velocity 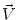 .
.











