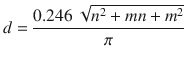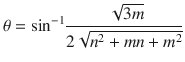1.1 Introduction
To understand the structure and properties of carbon nanotubes (CNTs), one needs to understand carbon chemical bonds . Carbon atoms can bond to other atoms via SP3, SP2, or SP covalent bonds. For example, carbon atoms are bonded via SP3 bonds (sharing their four valence electrons with four other carbon atoms) in diamond. As a result, diamond is one of natures hardest materials and has an ultrahigh thermal conductivity of ~1000 W/mK [].
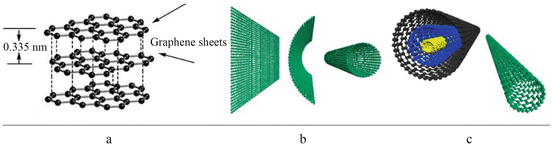
Fig. 1.1
Graphite, graphene [] and different types of carbon nanotube: single walled, double walled and multiwalled carbon nanotubes. ( a ) graphite consists of stacked graphene sheets. ( b ) a single walled carbon nanotube can be regarded as a rolled graphene sheet. ( c ) A multi-walled and a single walled carbon nanotube
CNTs were discovered in 1991 by Iijima [].
1.2 Structure and Properties of CNTs
1.2.1 CNT Structure
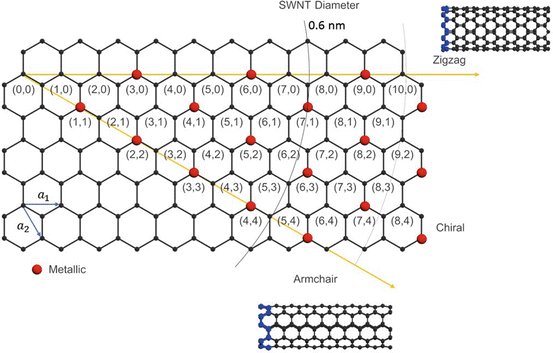
As shown in Fig. , a CNT can be described by two directional indices ( n , m ). Directional indices (also described by the chiral vector, na 1 + ma 2) determine the rolling direction of graphene to form a nanotube, as well as the diameter, d , of a nanotube:
Fig. 1.2
Chirality table for single-walled carbon nanotubes (SWCNTs)
The angle between the chiral vector ( n , m ) and the horizontal vector ( n , 0) in Fig. is known as the chiral angle:
The chiral angle takes a value between 0 and 30 for different types of nanotubes. Specifically, an armchair nanotube ( n = m ) has a chiral angle of 30, a zigzag nanotube ( n = 0, or m = 0) has a chiral angle of 0, and a chiral nanotube (any other n or m ) has a chiral angle between 0 and 30. The CNT bandgap varies by its chirality from 0 to 2 eV. The bandgap of semiconducting CNTs is inversely proportional to the nanotube diameter []. For example, all armchair nanotubes are metallic, whereas zigzag nanotubes for which n m 3 j exhibit semiconducting characteristics.
1.2.2 Electrical, Thermal, and Mechanical Properties
Ballistic transport is the ability of a material to transport electrons or phonons through its medium, with almost no resistance or scattering. Defect-free CNTs are very promising in electrical and thermal applications because of their ballistic transport ability over long lengthswith absence of electron/phonon scattering [].
While individual nanotube conductivities are surprisingly high, contact resistance between nanotubes, as well as between nanotubes and other materials, has made it difficult to translate individual nanotube properties to the micro- or macroscale. For example, for individual nanotubes with a conductivity of 3000 W/mK, one, two, and three-dimensional CNT networks have thermal conductivities of approximately 250, 50, and 3 W/mK, respectively [].
Mechanical properties of individual CNTs and their interactions have been investigated both numerically and experimentally. They have a Youngs modulus of up to 1.4 TPa, 2030% elongation to failure, and a tensile strength higher than 100 GPa [ shows two CNT fibers that are strong enough to hold a lamp while being electrically conductive enough to supply the electricity.
Fig. 1.3
Carbon nanotube (CNT) fibers support a lamp and supply the electricity. (Photo by Jeff Fitlow, courtesy of Matteo Pasqualis research group at Rice University)
1.3 CNT Synthesis
Different synthesis methods have been developed for CNTs. Over the years, these methods have been improved and optimized to allow for control of the diameter and chirality, length, number of walls, crystallinity, and impurity. Other attempts have been focused on scaling up CNT production and continuous CNT growth. CNTs can be synthesized by several techniques such as arc discharge [].
1.3.1 Carbon Arc Discharge
Iijima [] utilized an arc discharge method to synthesize CNTs [23], leading to their discovery. A direct current (DC) is run between two vertical electrodes, which are placed in a reaction tube, and an arc discharge is generated between the electrodes. In this method, the thin anode electrode has small holes filled with a mixture of graphite and powder metals . The mixture gets vaporized by the discharge at high temperatures (1200 C) and a flow of inert gas. SWCNTs grown using this method had an average diameter of 1 nm and were deposited on a collector downstream from the furnace. This method has the advantage of high yields but requires high energies and temperatures for sublimation of solid targets.
1.3.2 Laser Ablation of Carbon
Laser ablation is the process of evaporation or sublimation of a material heated by a laser beam. For synthesis of CNTs, Smalley et al. [] used laser ablation of graphite sources where carbon atoms were assembled into the form of nanotubes.
1.3.3 Flame Method
In this method, a hydrocarbon reacts with an oxidizer to produce a precursor mixture that gets deposited on catalyst particles as CNTs. The growth substrate is positioned inside the flame, and the flame provides the required energy for the process [].
1.3.4 Chemical Vapor Deposition
Its low cost (due to its relatively low operating temperatures), ability to control nanotube diameter and length, and scalability have made CVD the most readily available method for CNT synthesis. In CVD, nanotubes are grown from a catalyst particle. The catalyst is either on a substrate or formed in a gas flow inside a furnace. In particular, the precursor gases decompose, and CNTs are deposited on the catalyst particles. The precursor gases are usually a source of carbon (such as alcohols [] (generally used for mass production). Among these, WACVD seems to be a cost-effective method for large-volume production of high-quality CNTs.
Although many parameters affect CNT growth, the catalyst is known to have a significant impact. Thus, preparation of the catalyst becomes of great importance for controlling the final CNT structure. For growing vertically aligned CNTs on a substrate, catalyst nanoparticles should be prepared prior to CNT growth. A variety of methods have been tried for preparing catalysts on substrates, such as the sol-gel technique []. Reducing the two-step process of preparing the catalyst substrate and synthesis of CNTs to a one-step process can significantly improve the cost and rate of production. Reduction and breaking of precursor films into nanoparticles appears to be a promising approach for this purpose. Catalyst particles can also be generated in the growth tube. For example, in floating catalyst CVD, both the catalyst gas mixture and precursors are simultaneously introduced into the growth chamber. Catalyst nanoparticles are formed in the vapor phase, and nanotubes are grown from the catalysts and subsequently collected downstream.