INDEX
0.Research objectives
1.History of Nano-Materials
2.Nanomaterials
3.Carbon
4.Types, characteristics and applications4.1. Organic molecules
4.2.Carbon nanotubes
4.3.Nanowires
4.4.Quantum dots
5.Nanofabrication techniques
5.1 Top-Down
5.2 Bottom-Up
5.3 Chemical Synthesis
5.4 Positional Assembly
5.5 Self-Assembly
6.Conclusions
7.Bibliographic references and webgraphy used7.1 Reference Websites
0.RESEARCH OBJECTIVES
This brief investigation is made with the goal of getting to know the advances that have been made thanks to theScience and Engineering in the past years until the practical actuality. In this case, I will center my attention in thecalled Nanomaterials, and so establish the characteristics that they present, the ways of obtaining nanomaterialsand also their application in the electric and electronic sectors.
1.HISTORY OF NANOMATERIALSEverything began when Richard Feyman, Nobel Prize of Physics of 1965 made reference tothe possibilities of nanoscience and nanotechnology in a speech given in the Technology Institute of California (29 of December of1959) titled There's Plenty of Room at the Bottom. But Feyman didnt go deeper into his reflections and it wasnt until Eric Drexler MIT Student at the beginning of the 80s insinuated the possibility of creating Engineering systems at a molecular level. He published a book in 1986 titled Engines of Creation to describe the vision of Richard Feynman about the miniaturization at molecular scale. Nowadays, nanotechnology points to be the tool thatin short-term, will generate the new advances in Engineering and hence will be the first development engine of society, pretending to be and already being a place where new ideas converge for the development of the actual andfuture generations.
2.NANOMATERIALSAre those in which at least one of their dimensions can be found within the range of the nanoscale, namely, between1 and 100 nanometers. The most important and surprising quality of this new family of materials, is the development of important properties dependant of the size when its dimensions reach the nanometric range. The correctclassifications for the nanomaterials are divided in base of their dimensions or one of their components establishing that way three categories: OD, 1D and 2D. Inside a nanomaterial, the movement of the electrons is too limited by the dimensions of the own material. Also, the proportion of the atoms in the surface regarding the internal one is way higher than in materials of a bigger size. Therefore, if we reduce the dimensions of a material, we modify their properties and in consequence, we could design materials with properties on demand. (The carbon or graphite stops being that weak material at macromolecular level, to transform at a nano level into super-resistant nanotubes.)
The boom experienced by the research inside the field of nanomaterials in the past years puts on manifest thepotential applications of these materials in diverse sectors, both society and industry in terms of appearance or characteristics.
 3.CARBON
3.CARBONThe carbon is maybe the most important atom in our world. Even thought it only represents 1% of all the knownmatter of the universe and only 0,3% of the Earth's crust, it is the main component of living beings (20% mass). All the chemistry that gives life to the place is based in the carbon and it is known as organic chemistry. But, whatmakes carbon so special? The atom of carbon presents unique properties, being its chemistry much more extensive than the rest of the elements of the periodic table. Also the huge amount of different compounds that exist innature resulting of their combination through covalent bonds with a few more elements, results a bit curious how a single atom can give place to such different materials. Just think, for example, in the different properties that presents a diamond, graphite (like the mine of the pencil) or a piece of amorphous carbon. Three equal materials interms of composition, since they are all made exclusively by carbon atoms, but absolutely different between them.
To understand why, it is necessary to begin considering the electronic configuration of the carbon atom. Remember that this element possesses 6 electrons that, at the beginning, will distribute in the different atomic orbitals (energylevels permitted to the electrons in their movement around the nucleus) in the following way: 1s2 2s2 2p2. Now, thefour orbitals of the last level (called valence orbitals) dont stay the same but they can mix between them creating new orbitals called hybrid orbitals. Depending on how they do it, they give place to different types of orbitals inwhich the four valence electrons will be located; these are the ones that participate in the chemical bond to form different compounds. The types of possible hybridization to carbon are called sp3, sp2 or sp.
In the following table a summary of their characteristics can be seen:
Types of hybridizationResulting orbitals sp34 hybrid orbitals sp3sp23 hybrid orbitals sp2, 1 p pure
orbital
sp2 hybrid orbitals, 2 p pure
orbitals.
Resulting geometry (Angles) Tetrahedral (109 28)
Trigonal flat
Linear (180)
Therefore, the key in this case reside in that, even though that for example, diamond and graphite are exclusivelymade by the same element, the way that their atoms are arranged in the space is absolutely different and it is intimately bonded to the type of hybridization that has been produced between their orbitals. Thus, in the diamond the carbon atoms present a sp3 hybridization, which means four hybrid orbitals have been originated and they adopt a tetrahedral disposition in the space. Each carbon atom joins other four through a covalent bond giving place to thetridimensional structure that we have represented in the figure 1.1A. And it is exactly this tridimensional net of covalent bonds what determines the huge hardness that the diamond presents.
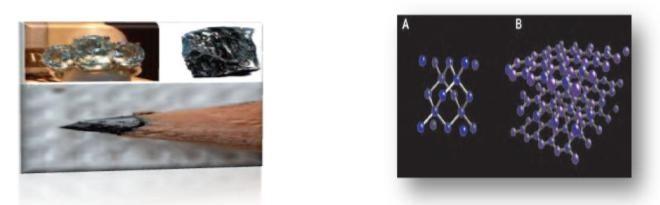
In fact, the origin of the word diamond has to been searched in a Greek term that means invincible. Among other properties that the diamond presents, and that makes it not only desired by its beauty but for its important technological properties, stands out its high melting point, its complete transparence and the fact that its not an electrical conductor but a thermal and electric isolator. The carbon with hybridization sp3 is also the most common in the biological molecules of which we will speak in chapter 5, in which the C is combined with otheratoms of C and also other elements like N, O, H, P, and S. However, when the carbon atoms present sp2hybridization, the hybrid orbitals distribute in a plane forming 120 angles (imagine the star of Mercedes cars or theblades of a wind turbine) and the orbital of p pure type is arranged perpendicular to the plane. This type of hybridization is the one that present the carbon atoms that form graphite, creating a material formed by the parallelplates. As we can see in the figure 1.1B, in the graphite each carbon atoms bonds strongly with other three carbon atoms creating a hexagonal net (like a bees panel). This type of materials present the particularity that even whenthe atoms are in the same plane they are strongly bonded between them (covalent bond between hybrids sp2), the plates bond to others through Van der Waals forces, that are weaker than covalent bonds. As an outcome, the properties of graphite are different in the plane and in the perpendicular direction. This means, the graphitepresents a great anisotropy in its properties; varying lots of them substantially depending on the direction it hasbeen pointed. From this characteristics of the atomic bond is derived its physical properties. Thereby, for example, thegraphite is a material that presents a high hardness in the plane but way less in the perpendicular direction and thereforeis very easy to separate it in sheets (Exfoliate it). Similarly, is a good electricity conductor in the plane, but very bad in theperpendicular direction. The graphite is also a very inert material. When the graphite is subjected to high temperaturesand pressures, it is possible to change its crystalline structure and it can become a diamond, as it happens inside the Earth.










 3.CARBON
3.CARBON