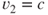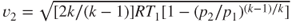WILEY END USER LICENSE AGREEMENT
Go to www.wiley.com/go/eula to access Wiley's ebook EULA.
APPENDIX 1
CONVERSION FACTORS AND CONSTANTS
Conversion Factors (arranged alphabetically)
Acceleration (L t2)
- 1 m/sec2 = 3.2808 ft/sec2 = 39.3701 in./sec2
- 1 ft/sec2 = 0.3048 m/sec2 = 12.0 in./sec2
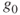 = 9.80665m/sec2 = 32.174ft/sec2 (standard gravity constant at Earth's surface)
= 9.80665m/sec2 = 32.174ft/sec2 (standard gravity constant at Earth's surface)
Area (L2)
- 1 ft2 = 144.0 in.2 = 0.092903 m2
- 1 m2 = 1550.0 in.2 = 10.7639 ft2
- 1 in.2 = 6.4516 104 m2
Density (M L3)
- Specific gravity is dimensionless, but has the same numerical value as density expressed in g/cm3; 1 liter = 103 m3
- 1 kg/m3 = 6.24279 102 lbm/ft3 = 3.61273 105 lbm/in.3
- 1 lbm/ft3 = 16.0184kg/m3
- 1 lbm/in.3 = 2.76799 104kg/m3
Energy, also Work or Heat (M L2t2)
- 1.0Btu = 1055.056J (joule)
- 1.0kWhr = 3.600 106J
- 1.0ftlbf = 1.355817J
- 1.0cal = 4.1868J
- 1.0kcal = 4186.8J
Force (M Lt2)
- 1.0lbf = 4.448221N
- 1dyne = 105N
- 1.0kg (force) [used in Europe] = 9.80665N
- 1.0 ton (force) [used in Europe] = 1000 kg (force)
- 1.0N = 0.2248089lbf
- 1.0 millinewton (mN) = 103 N
- Weight is the force acting on a mass being accelerated by gravity (
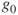 applies at the Earth's surface)
applies at the Earth's surface)
Length (L)
- 1m = 3.2808ft = 39.3701in.
- 1ft = 0.3048m = 12.0in.
- 1in. = 2.540cm = 0.0254m
- 1mile = 1.609344km = 1609.344m = 5280.0ft
- 1nautical mile = 1852.00m
- 1mil = 0.0000254m = 1.00 103in.
- 1 micron (m) = 106 m
- 1 astronomical unit (au) = 1.49600 1011 m
Mass (M)
- 1slug = 32.174lbm
- 1kg = 2.205lbm = 1000g
- 1lbm = 16ounces = 0.4536kg
Power (M L2t3)
- 1Btu/sec = 0.2924W (watt)
- 1J/sec = 1.0W = 0.001kW
- 1cal/sec = 4.186W
- 1horsepower = 550ftlbf/sec = 745.6998W
- 1ftlbf/sec = 1.35581W
Pressure (M L1 t2)
- 1bar = 105N/m2 = 0.10MPa
- 1atm = 0.101325MPa = 14.696psia
- 1mm of mercury = 133.322N/m2 (or Pa)
- 1MPa = 106N/m2
- 1 psi or lbf/in.2 = 6894.757N/m2
Speed (or linear velocity) (L t1)
- 1ft/sec = 0.3048m/sec = 12.00in./sec
- 1m/sec = 3.2808ft/sec = 39.3701in./sec
- 1knot = 0.5144m/sec
- 1mile/hr = 0.4770m/sec
Specific Heat (L2t2T1)
- 1gcal/gC = 1kgcal/kgK = 1Btu/lbmF = 4.186J/gC = 1.163 103kWhr/kgK
Temperature (T)
- 1K = (9/5)R = 1.80R
- 0C = 273.15K
- 0F = 459.67R
- C = (5/9)(F 32) and F = (9/5)C + 32
Time (t)
- 1mean solar day = 24hr = 1440min = 86, 400sec
- 1calendar year = 365 days = 3.1536 107sec
Viscosity (ML1t1)
- 1centistoke = 1.00 106m2/sec (kinematic viscosity)
- 1centipoise = 1.00 103kg/msec
- 1lbfsec/ft2 = 47.88025kg/msec
Constants
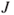 | Mechanical equivalent of heat = 4.186 joule/cal = 777.9 ftlbf/Btu = 1055 joule/Btu |
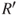 | Universal gas constant = 8314.3J/kgmolK = 1545ftlbf/lbmmolR |
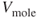 | Molecular volume of an ideal gas = 22.41 liter/kgmol at standard conditions |
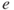 | Electron charge = 1.60218 1019coulomb |
| Permittivity of vacuum = 8.854187 1012 farad/m |
| Gravitational constant = 6.674 1011 m3/kgsec2 |
| Boltzmann's constant | 1.38065003 1023 J/K |
| Electron mass | 9.109381 1031 kg |
| Avogadro's number | 6.02252 1026/kgmol |
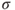 | StefanBoltzmann constant | 5.6696 108 W/m2K4 |
Notes
The letters in parentheses after each heading indicate the dimensional parameters (L = length, M = mass, t = time, and T = temperature).
APPENDIX 2
PROPERTIES OF THE EARTH'S STANDARD ATMOSPHERE
Sealevel pressure is 0.101325 MPa (or 14.696 psia or 1.000 atm).
| Altitude (m) | Temperature (K) | Pressure Ratio | Density (kg/m3) |
| 0 (sea level) | 288.150 | 1.0000 | 1.2250 |
| 1,000 | 281.651 | 8.8700 101 | 1.1117 |
| 3,000 | 268.650 | 6.6919 101 | 9.0912 101 |
| 5,000 | 255.650 | 5.3313 101 | 7.6312 101 |
| 10,000 | 223.252 | 2.6151 101 | 4.1351 101 |
| 25,000 | 221.552 | 2.5158 102 | 4.0084 102 |
| 50,000 | 270.650 | 7.8735 104 | 1.0269 103 |
| 75,000 | 206.650 | 2.0408 105 | 3.4861 105 |
| 100,000 | 195.08 | 3.1593 107 | 5.604 107 |
| 130,000 | 469.27 | 1.2341 108 | 8.152 109 |
| 160,000 | 696.29 | 2.9997 109 | 1.233 109 |
| 200,000 | 845.56 | 8.3628 1010 | 2.541 1010 |
| 300,000 | 976.01 | 8.6557 1011 | 1.916 1011 |
| 400,000 | 995.83 | 1.4328 1011 | 2.803 1012 |
| 600,000 | 999.85 | 8.1056 1013 | 2.137 1013 |
| 1,000,000 | 1000.00 | 7.4155 1014 | 3.561 1015 |
Source: U.S. Standard Atmosphere, National Oceanic and Atmospheric Administration, National Aeronautics and Space Administration, and U.S. Air Force, Washington, DC, 1976 (NOAAS/T1562).
APPENDIX 3
SUMMARY OF KEY EQUATIONS FOR IDEAL CHEMICAL ROCKETS
| Parameter | Equations | Equation Numbers |
Average exhaust velocity, 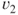 (m/sec or ft/sec) (assume that (m/sec or ft/sec) (assume that 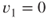 ) ) | 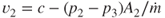 | 215 |
When 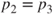 , , 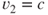 |
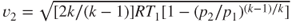 |
Next page












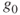 = 9.80665m/sec2 = 32.174ft/sec2 (standard gravity constant at Earth's surface)
= 9.80665m/sec2 = 32.174ft/sec2 (standard gravity constant at Earth's surface)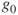 applies at the Earth's surface)
applies at the Earth's surface)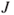
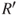
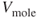
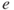
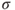
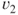 (m/sec or ft/sec) (assume that
(m/sec or ft/sec) (assume that 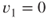 )
)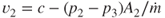
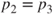 ,
,