Springer-Verlag GmbH Germany 2017
Qing Li and Yiu-Wing Mai (eds.) Biomaterials for Implants and Scaffolds Springer Series in Biomaterials Science and Engineering 10.1007/978-3-662-53574-5_1
1. Multiscale Modelling and Simulation of Musculoskeletal Tissues for Orthopaedics
Clayton J. Adam 1
(1)
School of Chemistry, Physics and Mechanical Engineering, Faculty of Science and Engineering, Queensland University of Technology, GPO Box 2434, 2 George St, Brisbane, Q4000, Australia
Abstract
Successful development of implants for orthopaedic surgical procedures depends on a comprehensive understanding of the interaction between implant biomaterials and the host tissues of the musculoskeletal system. Mechanical factors are a key part of this interaction. This chapter investigates the potential of computational modelling and simulation approaches at multiple length scales to elucidate the response of musculoskeletal tissues to orthopaedic implants, leading to improved treatment outcomes. To a large extent, musculoskeletal tissues derive their load-bearing function from their hierarchically arranged structure; therefore we pay particular attention to modelling of the musculoskeletal tissues themselves from the nano- and micro-scales to the macro-scale. The most well-characterised musculoskeletal tissue are bone, and this chapter covers recent work on micro- and nanoscale modelling of bone and collagen and on defining the elasto-plastic constitutive response of the bone extracellular matrix using finite element models of bone nanoindentation combined with atomic force microscopy to map the inelastic deformation of the tissues. The use of serial milling and block face imaging techniques to determine soft tissue anatomy at multiple length scales for definition of model geometry is also covered. The potential for further development of multiscale computational biomechanics through modelling cell and tissue mechanobiology is discussed, including more detailed mechanical characterisation of the tissueimplant interface, modelling the strain fields experienced by cells within the extracellular matrix and within scaffolds and coupled modelling of other physical and biological phenomena within tissues and biomaterials.
1.1 Introduction
In the same way that computational mechanics has revolutionised the design and analysis of manufactured objects in many industries, the rapidly growing subfield of computational biomechanics (CB) offers much promise in using modelling and simulation to better understand degenerative processes in the musculoskeletal system and to improve orthopaedic treatments for musculoskeletal disorders. Since the function of the musculoskeletal system is predominantly a load-bearing one, it follows that the mechanics of interaction between an implant and its surrounding host tissues are of key importance in the design of orthopaedic implants and surgical procedures. This holds true both at the scale of whole bones, joints and implants (here designated the macro-scale) and at the micro- and nanosized scales of the implant surface and its mechanical interaction with hierarchically structured host tissues. From a biological perspective, the microenvironment within tissues and at the interface between tissues and implants is extremely important because this is the scale at which cells sense and respond to mechanical signals to control gene expression for tissue growth, repair and remodelling.
A key advantage of mechanics-based modelling in orthopaedic biomechanics is the ability to model many different design variants of an implant, with the aim of finding optimal design characteristics of that implant. This would be impossible in human trials and would be prohibitively time consuming and expensive in either in vitro or in vivo animal studies. Models also allow the same implant design to be simulated following virtual placement into a wide variety of different patients. A third possibility in computational biomechanics, and the focus of this chapter, is the ability to model biomechanical processes over a range of length and timescales, providing insights into the coupling between macro-scale loading and the micro- and nano-mechanical processes occurring within the tissues themselves. These micro- and nanoscale physical processes are the drivers of the biological response to mechanical loading and therefore affect the responses of the musculoskeletal system to orthopaedic implants. To date, cell-mediated processes such as tissue modelling and remodelling have been treated phenomenologically in macro-scale simulations of tissue response to implants; however, modelling at finer scales has the potential to provide a deeper physics-based mechanistic understanding of tissue response to loading.
With these considerations in mind, this chapter highlights the use of biomechanical modelling and simulation approaches to investigate the loading response of musculoskeletal tissues and orthopaedic implants at multiple scales. It is important to note that although the biomaterials used in an orthopaedic implant may themselves be intricate in geometry and surface topography, the much greater challenge is modelling host tissue responses to loading, because natural tissues are much more sophisticated than their synthetic replacements []. Therefore the focus of this chapter is on modelling of tissues as natural biomaterials rather than modelling the metals, polymers and ceramics used for implants or scaffolds. Furthermore, the focus here is on the passive osseo-ligamentous tissues, bone, cartilage, ligament and tendon, i.e. excluding the muscle. While many of the considerations that follow are also relevant to muscle, its active contractile force-generating ability introduces additional complexities in modelling which are beyond the scope of this discussion.
It is also important to note that due to the complexities of modelling living tissues [], but must necessarily come after efforts to define and model the scales of relevance for cellular and extracellular matrix loading response.
1.1.1 Orthopaedic Implants and Treatment Outcomes
The aim of orthopaedic surgery is to correct problems which arise in the human musculoskeletal system. Disorders of the musculoskeletal system are a major contributor to the global burden of disease, and there is intense interest in developing new treatments for musculoskeletal disease. In childhood, bone deformities during growth can require major corrective surgery. In adults, disorders such as osteoarthritis and intervertebral disc degeneration cause immobility, pain and lost productivity. In the elderly, osteoporosis causes increased fracture risk with subsequent loss of mobility and high mortality rates after a fracture. At all ages, cancer, trauma and infectious disease can lead to bone, joint and soft tissue destruction.
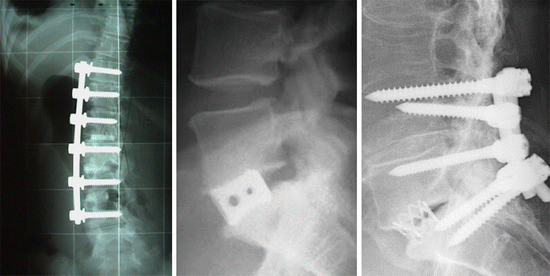
The dominant paradigm in orthopaedic practice today is the use of non-biodegradable implants which may have various surface treatments or coatings applied to enhance osseointegration with host bone (Fig. ), although we note that there is also intensive research underway in the field of tissue engineering, in which the implanted load-bearing structure is in fact a temporary scaffold which is designed to resorb in the body and eventually be replaced by host tissue.