1. Introduction: Fundamentals of Nuclear Physics
1.1 Natural Stable and Radioactive Isotopes
It was assumed during ancient times and up to the end of the nineteenth century that the smallest particle of an element is an atom, which was the main subject of study in atomic physics. But as time passed, a magic 18951905 decade appeared in physics.
In 1895 German physicist W. Roentgen discovered X-rays produced by the Crookes cathode tube . In 1896 French physicist A. Becquerel discovered the natural radioactivity of uranium decayed products. In 1897 English physicist J. Thompson in the Cavendish laboratory identified the electron , the fundamental particle of matter. In 1898 Pierre and Maria Curie discovered radioactivity of polonium and radium . Later on, in addition to the elements of the thorium, uranium and actinium series, natural radioactivity was discovered for kalium-40, rubidium-87, samarium-82 and others, which did not belong to the trans-uranium series.
In 1934 F. and I. Joliot-Curie first obtained artificial radioactive elements. In 1911, in experiments on scattering of -particles by a matter, E. Rutherford discovered the atomic nucleus and in 1918 he identified the proton. He observed scattering of -particles by two large angles and assumed that the charge of an atom is located in its centre. The fact of existence of the atomic nucleus was not immediately accepted. Only after N. Bohr introduced his quantum theory of the atom and G. Moseley experimentally showed the shift of the dark-line roentgen spectrum of different atoms was the atomic nucleus considered in physics. Later on in 1934 the neutron was identified by G. Chadwick. However, even today, an acceptable theory of the atomic nucleus structure does not exist.
In 1913, English radio-chemist F. Soddy proposed the term isotopes for identifying atoms of the same element but having different masses. It was done in order to determine places of the decay products of the radioactive elements in Mendeleevs table of chemical elements. The famous English physicians J. Thomson and F. Aston played an important role in the discovery and study of stable isotopes. In 1911, Thompson developed the method of parabolas for determination of the ratio of the particle charge to its mass by recording mass spectra. Based on this, using the apparatus designed in the Cavendish Laboratory, Thomson discovered the neon isotopes of mass number 22, which was reported in January 1913. In 1919, Aston proved the existence of the neon isotopes and soon found the isotopes of chlorine and mercury. Several years later, more than 200 isotopes of various elements were discovered, with the exception of those of hydrogen and oxygen that were then considered as simple elements.
In 1929, W. Giauque and H. Johnston, applying the new techniques of research by absorption air spectra, discovered the isotopes of oxygen of mass numbers 17 and 18. In 1931, Johnston, on the basis of the free electrons and protons rule developed from analysis of established isotopes, concluded that hydrogen isotopes with masses 2 and 3 should exist. Soon after this in 1932, Urey, Brickwedde and Murthy, using the mechanism of isotope fractionation , detected the Balmers lines of deuterium in the enriched spectrum of gaseous hydrogen using the spectral method. A detailed history of isotope discovery and isotope studies has been reported by Aston in his monograph (Aston ).
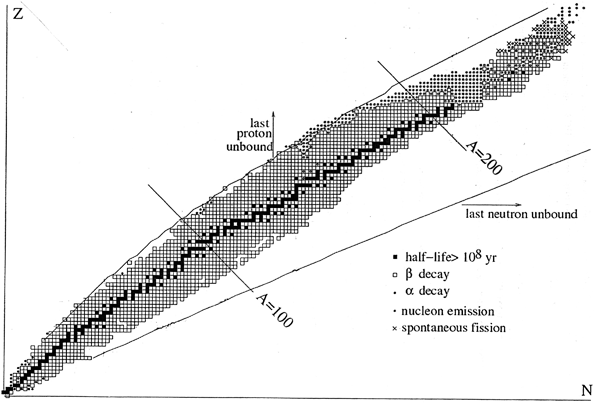
Atoms are the smallest amount of matter that retain the properties of an element. Atoms are composed of smaller particles that are protons, neutrons and electrons. At present, there are 105 different elements on the Earth, 90 of them exist in nature and 15 are man-made. The total number of protons of an atom is equal to the number of electrons and is given the symbol Z. The number of non-charged neutrons in a nucleus is given the symbol N. The mass number of the nucleus is the total number of protons and neutrons, which is given the symbol A=Z+N. Each of the chemical elements has a unique atomic number because the atoms of different elements contain a different number of protons and a different combination of protons and neutrons. Not all combinations of numbers of protons and neutrons are possible. Up to now, about 2500 specific nuclides with unique combinations of neutrons and protons have been identified in the solar-system matter. Figure shows the general picture of the world of nuclei .
Fig. 1.1
A map of nuclei (Basdevant et al. )
The long-lived nuclei present on the Earth are shown by black squares Unbounded combinations of N and Z lie outside the lines marked last proton/neutron unbound.
The majority of combinations of the protons and neutrons appear to form unstable nuclei , which are therefore short-lived or not formed at all. It is assumed in astrophysics that the pre-solar cloud, from which ~4.5 billion years ago the Sun, the planets, satellites and meteorites were formed, initially consisted of 1H (~75%) and 4He (~25%). These two elements were formed in the primordial Universe during the big bang at the cosmologic temperature kT=60 keV. All other elements, which are ~2%, were formed in the stars by nucleosynthesis. Namely, elements with A<60 were formed by 4He burning at exothermic reactions. As a result, peaks in solar-system abundances are observed for the elements 4He, 12C, 16O, 20Ne, 28Si, 32S, 36Ar, 40Ca and 56Fe. And creation of elements with A >60 is assumed to have occurred at the star nucleosynthesis by slow and fast neutron capture . The number of neutrons in a nucleus with Z protons can be in some limit different because the nucleus stability is determined by the proton-neutron ratio. The elements with odd Z do not have more than two stable isotopes . The elements with even Z do have more than two stable isotopes. Tin has 10 (maximum) stable isotopes, xenon has 9 and cadmium and tellurium have 8 stable isotopes. The content of the isotopes in the Earths crust is practically limited by 10 elements from 8 to 26 Z (Table ).
Table 1.1
Stable isotopes composing the Earths crust
Element | Atomic number, Z | Mass content, % |
|---|
O | | 47.0 |
Si | | 29.5 |
Al | | 8.05 |
Fe | | 4.65 |
Ca | | 3.30 |
Na |