Richard Wolfson - Nuclear Choices for the Twenty-First Century: A Citizens Guide
Here you can read online Richard Wolfson - Nuclear Choices for the Twenty-First Century: A Citizens Guide full text of the book (entire story) in english for free. Download pdf and epub, get meaning, cover and reviews about this ebook. year: 2021, publisher: MIT Press, genre: Romance novel. Description of the work, (preface) as well as reviews are available. Best literature library LitArk.com created for fans of good reading and offers a wide selection of genres:
Romance novel
Science fiction
Adventure
Detective
Science
History
Home and family
Prose
Art
Politics
Computer
Non-fiction
Religion
Business
Children
Humor
Choose a favorite category and find really read worthwhile books. Enjoy immersion in the world of imagination, feel the emotions of the characters or learn something new for yourself, make an fascinating discovery.

- Book:Nuclear Choices for the Twenty-First Century: A Citizens Guide
- Author:
- Publisher:MIT Press
- Genre:
- Year:2021
- Rating:5 / 5
- Favourites:Add to favourites
- Your mark:
- 100
- 1
- 2
- 3
- 4
- 5
Nuclear Choices for the Twenty-First Century: A Citizens Guide: summary, description and annotation
We offer to read an annotation, description, summary or preface (depends on what the author of the book "Nuclear Choices for the Twenty-First Century: A Citizens Guide" wrote himself). If you haven't found the necessary information about the book — write in the comments, we will try to find it.
Nuclear Choices for the Twenty-First Century: A Citizens Guide — read online for free the complete book (whole text) full work
Below is the text of the book, divided by pages. System saving the place of the last page read, allows you to conveniently read the book "Nuclear Choices for the Twenty-First Century: A Citizens Guide" online for free, without having to search again every time where you left off. Put a bookmark, and you can go to the page where you finished reading at any time.
Font size:
Interval:
Bookmark:

Nuclear Choices for the Twenty-First Century
A Citizens Guide
Richard Wolfson and Ferenc Dalnoki-Veress
The MIT Press
Cambridge, Massachusetts
London, England
2021 Massachusetts Institute of Technology
All rights reserved. No part of this book may be reproduced in any form by any electronic or mechanical means (including photocopying, recording, or information storage and retrieval) without permission in writing from the publisher.
Library of Congress Cataloging-in-Publication Data
Names: Wolfson, Richard, author. | Dalnoki-Veress, Ferenc, author.
Title: Nuclear choices for the twenty-first century : a citizens guide / Richard Wolfson and Ferenc Dalnoki-Veress.
Description: Cambridge, Massachusetts : The MIT Press, 2021. | Includes bibliographical references and index.
Identifiers: LCCN 2020025433 | ISBN 9780262542036 (paperback)
Subjects: LCSH: Nuclear energy--Popular works.
Classification: LCC TK9145 .W592 2021 | DDC 621.48--dc23
LC record available at https://lccn.loc.gov/2020025433
d_r0
List of Figures
(a) A truckload of uranium fuel arrives at the Vermont Yankee nuclear power plant. Four such truckloads supplied all the fuel needed for the now-closed plants once-in-18-months refueling (Entergy Nuclear). (b) A 110-car trainload of coal arrives at a Kansas power plant. Fourteen such trainloads fuel the plant each week (Earl Richardson, Topeka Capital-Journal). The contrast between figures 2.1a and 2.1b is a manifestation of the nuclear difference.
Three particles and the forces by which they interact.
The hydrogen nucleus is a single proton. Other simple nuclei are deuterium (one proton and one neutron, also called a deuteron), tritium (one proton, two neutrons), helium-3 (two protons, one neutron), and helium-4 (two protons, two neutrons).
Atoms of hydrogen and helium. Note that in each case the number of orbiting electrons is the same as the number of protons in the nucleus.
A chemical reaction. Here one atom of carbon joins two atoms of oxygen to form carbon dioxide. This is the basic reaction involved in burning coal. The energy released is 4.1 in units used by physicists working with atomic and nuclear processes. The black dots represent atomic nuclei, which arent affected by the rearrangement of the atoms.
A nuclear reaction. Here two deuterium nuclei join to form a helium-4 nucleus. The nuclei are less than 1/10,000 the size of the atoms in figure 2.5, but the energy released is some 6 million times that of the chemical reaction shown in the preceding figure.
Some nuclei with element names and symbols. Figures are only suggestive; nucleons arent locked into the fixed patterns shown here.
Isotopes of a given element have the same number of protons but different numbers of neutrons. Shown are three isotopes of hydrogen and two each of helium, oxygen, and uranium. Although isotopes have similar chemical behavior, their nuclear behavior can be very different. For example, only the rare isotope uranium-235 can serve directly as fuel for nuclear reactors and weapons.
Anatomy of a nuclear symbol.
Two widely separated protons in a large nucleus experience mutual repulsion due to the long-range electric force. But because of its short range, the attractive nuclear force between them is insignificant. An excess of neutrons is therefore necessary to hold the nucleus together.
Neutron number versus proton number for stable nuclei, represented by small black squares. Most smaller nuclei have equal or nearly equal numbers of protons and neutrons, but larger nuclei have many more neutrons, for the reason explained in figure 2.10. Inset is a magnification showing the first four stable isotopes.
Marie and Pierre Curie in their Paris laboratory. (Science Source)
Alpha decay of uranium-238 yields an alpha particle (helium-4 nucleus) and thorium-234.
Beta decay of carbon-14. A neutron in C-14 turns into a proton and an electron; the electron is ejected, leaving nitrogen-14. Note that the mass number remains unchanged (6 + 8 for C-14 and 7 + 7 for N-14).
An excited nucleus (arrows suggest vibration) sheds excess energy by emitting a gamma ray (wavy line). No nucleons or electric charge leave the nucleus, so it retains its identity.
(a) Simplified diagram of a Geiger counter; (b) a Geiger counter in use. (Hank Morgan/Science Source)
Decay of a radioactive material. In one half-life, half the nuclei still present will decay.
The decay of uranium-238 results in shorter-lived nuclei that are found wherever uranium is present. Here the decay chain is shown on a chart of mass number versus atomic number and is carried as far as radon-222. The chain continues until it reaches the stable isotope lead-206.
(a) Sources of background radiation for the average human. Natural sources, especially radon-222, dominate. The average yearly dose is about 3 millisieverts. (b) In the United States just over half the background radiation is from artificial sources, especially medical procedures. The U.S. average yearly dose is some 6 mSv, twice the global average. (Data source: UN Scientific Committee on the Effects of Atomic Radiation)
Excess solid cancers as a percentage of expected cancer incidence in Japanese bomb survivors, as a function of dose in grays (Gy). (Data source: D. L. Preston et al., Solid Cancer Incidence in Atomic Bomb Survivors: 19581998, Radiation Research 168, no. 1 [July 2007], Table 9.)
Dose-response curves corresponding to different hypotheses about the health impact of low radiation doses. Curve A indicates higher risk at low doses. Curve B is the linear no-threshold model. Curve C shows lower risk at low doses, and curve D is a hormesis model with a threshold below which radiation is actually beneficial. All are in essential agreement at higher doses (above 50 mSv), where health impacts become increasingly well verified.
Patient being prepared for particle-beam radiation treatment. (National Cancer Institute/Science Source)
Scan of a human brain using positron emission tomography (PET). (Steven Needell/ Science Source)
Diagram showing how a neutron source is used to probe the geology surrounding an oil well. High-energy neutrons from the source scatter off the surrounding rock, generating additional thermal neutrons and some gamma rays. Detectors sense these particles, yielding information about the rock structure. Neutrons interact most readily with hydrogen, making the technique especially useful for determining porosity of the rockan indicator that oil might be present.
Food preservation by irradiation. The potato at left is eight months old and was untreated. The potato at right is the same age but received 200 Gy of radiation. (Brookhaven National Laboratory)
Radiocarbon dating. (a) Carbon-14, formed in the atmosphere by cosmic rays, is incorporated into living things through the food chain. As a result, living organisms maintain a mild but steady level of C-14 radioactivity. (b) At death, C-14 uptake ceases. (c) Much later, C-14 activity has decreased substantially. (d) Archeologists excavate the long-dead remains. By measuring C-14 activity, they infer the time since death. Note that the archeologists, with their active C-14 intake, are more radioactive than their ancient ancestor. In practice, the radioactivity measurement is done not in the field but in the laboratory. (Artwork by Robin Brickman.)
The curve of binding energy. The graph plots binding energy per nucleon versus nuclear mass number. Near the top of the curve is iron-56 (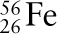
Font size:
Interval:
Bookmark:
Similar books «Nuclear Choices for the Twenty-First Century: A Citizens Guide»
Look at similar books to Nuclear Choices for the Twenty-First Century: A Citizens Guide. We have selected literature similar in name and meaning in the hope of providing readers with more options to find new, interesting, not yet read works.
Discussion, reviews of the book Nuclear Choices for the Twenty-First Century: A Citizens Guide and just readers' own opinions. Leave your comments, write what you think about the work, its meaning or the main characters. Specify what exactly you liked and what you didn't like, and why you think so.







