Jack Challoner - The atom : a visual tour
Here you can read online Jack Challoner - The atom : a visual tour full text of the book (entire story) in english for free. Download pdf and epub, get meaning, cover and reviews about this ebook. year: 2018, publisher: Ivy Press, The, genre: Romance novel. Description of the work, (preface) as well as reviews are available. Best literature library LitArk.com created for fans of good reading and offers a wide selection of genres:
Romance novel
Science fiction
Adventure
Detective
Science
History
Home and family
Prose
Art
Politics
Computer
Non-fiction
Religion
Business
Children
Humor
Choose a favorite category and find really read worthwhile books. Enjoy immersion in the world of imagination, feel the emotions of the characters or learn something new for yourself, make an fascinating discovery.

- Book:The atom : a visual tour
- Author:
- Publisher:Ivy Press, The
- Genre:
- Year:2018
- Rating:4 / 5
- Favourites:Add to favourites
- Your mark:
- 80
- 1
- 2
- 3
- 4
- 5
The atom : a visual tour: summary, description and annotation
We offer to read an annotation, description, summary or preface (depends on what the author of the book "The atom : a visual tour" wrote himself). If you haven't found the necessary information about the book — write in the comments, we will try to find it.
The atom : a visual tour — read online for free the complete book (whole text) full work
Below is the text of the book, divided by pages. System saving the place of the last page read, allows you to conveniently read the book "The atom : a visual tour" online for free, without having to search again every time where you left off. Put a bookmark, and you can go to the page where you finished reading at any time.
Font size:
Interval:
Bookmark:

THE BUILDING BLOCK OF EVERYTHING
JACK CHALLONER

From a distance, matter looks smooth and continuous. A solid object, such as a table, has definite edges and no apparent gaps in its structure; water spills from a cup as self-contained fluid streams and droplets; and the air we breathe, although invisible, feels like one gassy continuum. But at a scale far too small for us to truly comprehend, matter is bumpy and discontinuous; it is made up of empty space punctuated by countless tiny particles. This notionthat matter is made of extremely small particlesis called atomism. This book is a celebration of atomism, and it sets out some of the remarkable insights an atomic perspective has revealed about the world around us.
Despite the title of this book, and despite what the paragraph on the previous page might suggest, the atom is not actually the fundamental building block of matter. There are two reasons for this. First, an atom is itself made up of smaller particles: protons, neutrons, and electrons. Second, most matter is not actually made of atoms. The precise definition of an atom is of an isolated, self-contained object that has exactly the same number of protons as electronsa situation that rarely exists in the real world. Strictly speaking, nearly all the substances around us are made of molecules or ions, not atoms. A molecule is made of two or more atoms joined together, but the atoms are entwined, sharing their electrons, not isolated and self-contained. An ion has unequal numbers of protons and electrons, so is not an atom either. Nevertheless, it is convenient to speak of matter as being made of atoms. The atom is also a particularly useful archetype, a perfect starting point for understanding how matter works.
People have probably wondered what matter is made of for as long as they have wondered about anything. The modern definition of the atom has developed as a result of theory, observation, and experiment over the past two hundred years, however, the idea that matter is made of extremely small particles is much olderthough it has not always held sway. In the first chapter, we look back at the long history of the concept of atoms, providing an overview of centuries of inspired work by brilliant minds.
The modern understanding of how atoms behave depends upon quantum theory, a counterintuitive but well-tested set of rules that govern the interactions of particles at the atomic scale. explores quantum theory in some depth to make sense of the atoms basic structure. In this, its electrons are arranged in particular patterns around a dense central core, the nucleus, which is composed of the atoms protons and neutrons.
Only about ninety different kinds of atom exist naturally. Each has a different number of protons in the nucleus (always equal to the number of electrons arranged around it). Each kind of atom is called an element. A few elements were created in the first few minutes of the universe, as protons and neutrons bound together to form simple nuclei. Under the intense pressures and at the high temperatures inside stars, nuclei of these primordial atoms combine, or fuse, to form larger nuclei, the basis of heavier elements. Supernovasthe extremely energetic explosions that befall large stars when they reach the end of their existencecreate still heavier elements, because larger nuclei fuse together. explains these processes that brought the elements into existence and explores the elements properties. This chapter also introduces the periodic table, a way of organizing the elements into groups with similar properties.
explores how the interactions between atoms can help to explain the physical and chemical properties of matter. The interactions of large numbers of particles (atoms, molecules, or ions) can explain the physical properties and behaviors of solids, liquids, and gases, such as air pressure, evaporation, and the existence of surface tension. Attractive forces between atoms create bonds: chemical bonds that produce chemical compounds. So, for example, atoms of hydrogen and oxygen bond together to form the chemical compound we call water.
In explores some of the contributions that atomic theory have made to everyday life and beyond in the twentieth and twenty-first centuries. Quantum theory has, for example, given electronics engineers dominion over electrons, and that has led to the development of computers and other devices behind the digital revolution. Understanding nuclear reactions and radioactivity has opened up an enormous source of energy, which can be used for purposes both peaceful and otherwise.
The final chapter of this book, , reviews the current state of atomism, which is expressed in the Standard Model of particles and their interactions. This beautiful theory is the culmination of decades of work by theoretical and experimental physicists using powerful particle accelerators, such as the Large Hadron Collider at CERN on the SwissFrench border. The Standard Model makes sense of the huge menagerie of subatomic particles that exist and has made bold predictions that have been realized, such as the existence of the so-called God particle, the Higgs boson. At its heart are the true atoms: the fundamental (un-splittable) particles from which the world is made. Within the Standard Model lies a conundrum, however, for it is based on quantum field theoryan extension of quantum theory in which particles are not solid objects, but instead manifestations of fields that permeate all of space. According to modern physics, then, atoms are nothing but waves drifting on an infinite, invisible, immaterial sea of potentiality.
the precise definition of an atom is of an isolated, self-contained object that has exactly the same number of protons as electrons
The idea that matter is made of tiny particles is at least 2,500 years old. Through much of history, it was relegated to the fringes of scientific thought for philosophical and religious reasons. But it regained popularity with the rise of science in Europe in the seventeenth and eighteenth centuries. The notion that atoms really do exist only became widely accepted with the rapid rise of atomic physics in the early twentieth century.
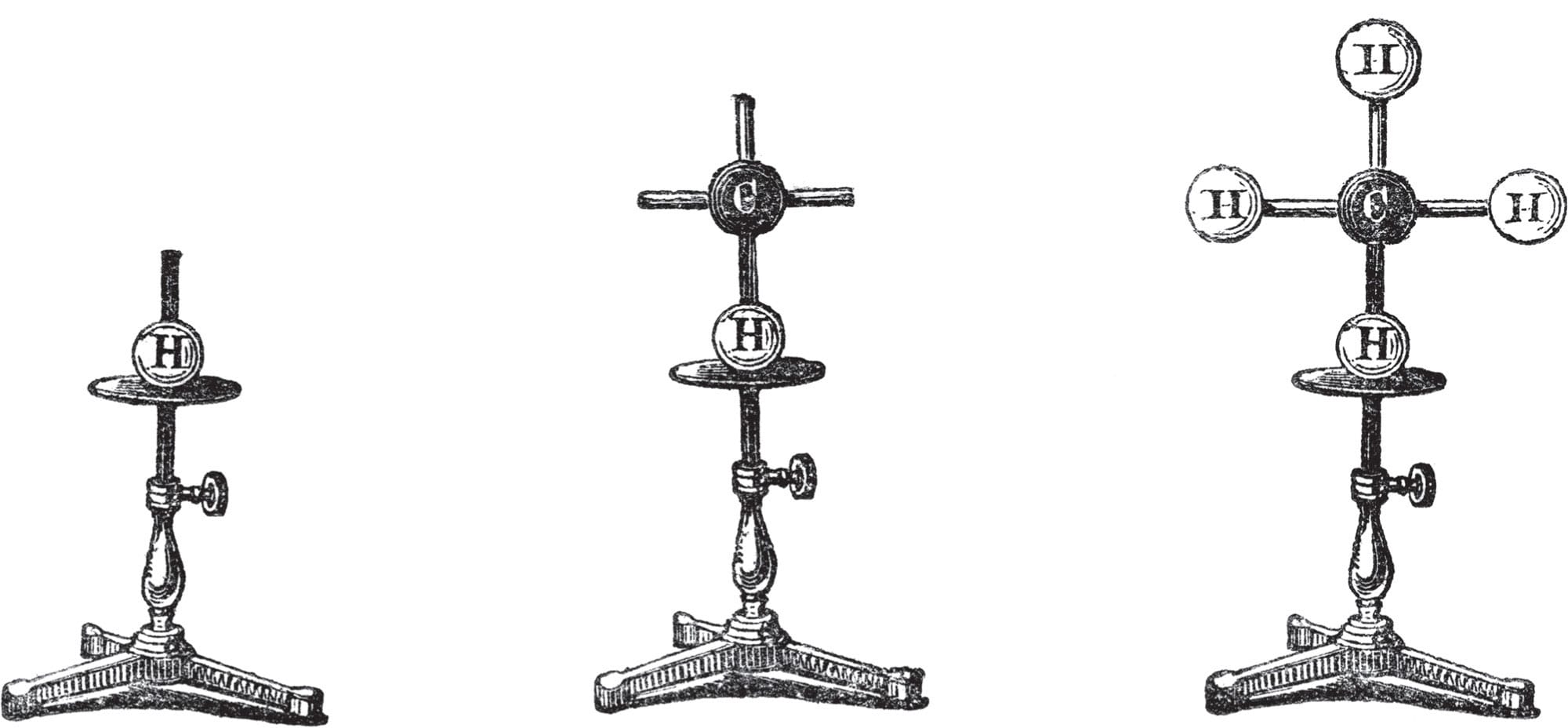
Molecular models presented to the Royal Institution, London, UK, by German chemist August Wilhelm von Hofmann, in 1865. Hoffman used the models in a lecture entitled The Combining Power of Atoms, at a time when the existence of atoms was still in doubt.
The roots of modern atomic theory lie in ancient Greece. Oddly, perhaps, the story begins with philosophical wrangling about whether change is real or an illusionand whether empty space can exist. Despite the development of well-thought-out and convincing atomic theories in both Greece and India, other ideas would come to dominate.
Font size:
Interval:
Bookmark:
Similar books «The atom : a visual tour»
Look at similar books to The atom : a visual tour. We have selected literature similar in name and meaning in the hope of providing readers with more options to find new, interesting, not yet read works.
Discussion, reviews of the book The atom : a visual tour and just readers' own opinions. Leave your comments, write what you think about the work, its meaning or the main characters. Specify what exactly you liked and what you didn't like, and why you think so.