Microorganisms constitute two of the three domains of life on the Earth. Microbiota plays a critical role in the ecosystem and human health. They exhibit huge biodiversity and metabolic versatility that allows them to abide and survive even in the harshest environmental conditions, making them all ubiquitous and be efficient and inexhaustible tools for the discovery of novel bioactive molecules. Although they possess ample potential, about 99% of the microbial world remains to be discovered. The primary explanation for this is that in vitro cultivation of these microorganisms remains fractious. The study of microbial heterogeneity and population dynamics has fundamentally been reshaped by the development of culture-independent techniques. The use of omics approaches by genomics, metagenomics, transcriptomics, proteomics, and metabolomic techniques contributes significantly to the discovery and characterization of a real microbial community. These omic techniques can revolutionize biological treatment in environmental engineering through extremely sensitive characterization of already uncultured microorganisms. As an environmentally friendly and economical replacement to minimize the load of pollution, biological approaches using microbes are gaining importance. New omic technologies such as metagenomics, metatranscriptomics, metaproteomics, and metabolomics now have indeed been utilized to develop methods to examine ecology and microorganism heterogeneity and to discover novel microorganisms for use in bioaugmentation, environment surveillance, and bioremediation. To obtain an accurate picture of living microorganisms, convergence of various layers of data, the multi-omics strategy, is therefore necessary. In this chapter, we present the evolving approaches and advanced tools for microbial population evaluation that support and extend conventional metagenomic profiling. They comprise measuring omic data types like metabolomics, proteomics, transcriptomics, genomics, and integrating various forms of multi-omic data in a combined framework for characterization of environmental microorganisms.
Keywords: Omics, genomics, proteomics, transcriptomics, metabolomics, microorganisms,
1.1 Introduction
In the field of biology, the word ome usually refers to the totality or entirety of a set of unique things. For example, a biome is a set of living organisms, and a genome is a collection of genes within a single organism. Omics, then, are fields of study which deal with these collections and require the characterization and consideration of numerous molecules concurrently []. The omics theory, however, goes far beyond DNA and may include transcripts of RNAs, proteins, and metabolites, also referred to as omics cascades. And in this cascade:
the genome (or metagenome) provides details about what could happen (i.e., the functional potential);
the transcriptome (or metatranscriptomic) provides data about what happens to be occurring (i.e., which genes are all being expressed);
the proteome (or metaproteome) provides knowledge about molecules that actually make things happen;
the metabolome provides details on what has already happened or is occurring currently [].
Metagenomics is designed to research organisms whose genetic materials could be specifically evaluated from samples retrieved from the field, while transcriptomics and proteomics are indeed a study of the function, components, and structure of various RNA and protein molecules []. In this chapter, we discuss the main omics-based approaches commonly used to characterize environmental microorganisms, and also the methods for analyzing and interpreting the bioinformation which these studies produce.
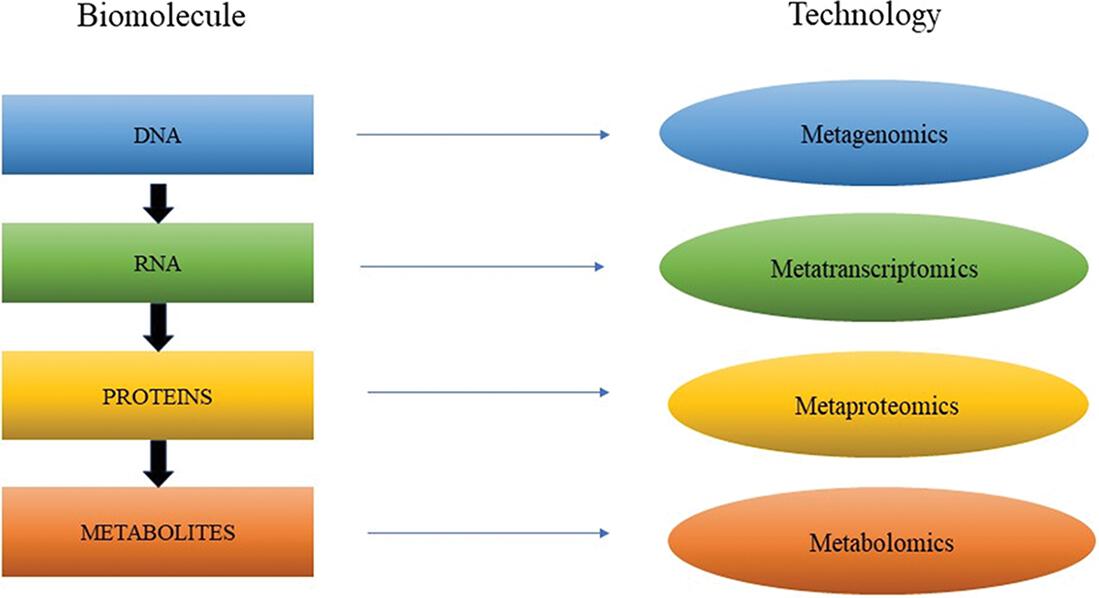
Figure 1.1: Different types of omics technique for microbial community analyses.
1.2 Metagenomics
The scientific community has extended the concept of metagenomics to generally include any technique centered on analyzing DNA derived from environmental samples [1]. Metagenomic-based assays have become the standard for characterizing microbial communities over the past two decades and have been used in numerous studies to investigate the structure, function, and metabolic capacity of microbial communities in a wide range of environments [1]. Due to the heterogeneity of microbial communities in these habitats, and the lack of a good collection of reference sequences from a broad microbial community to function as a scaffold for assembling the sequences, the integration of metagenomics data from environmental samples is extremely challenging currently [.
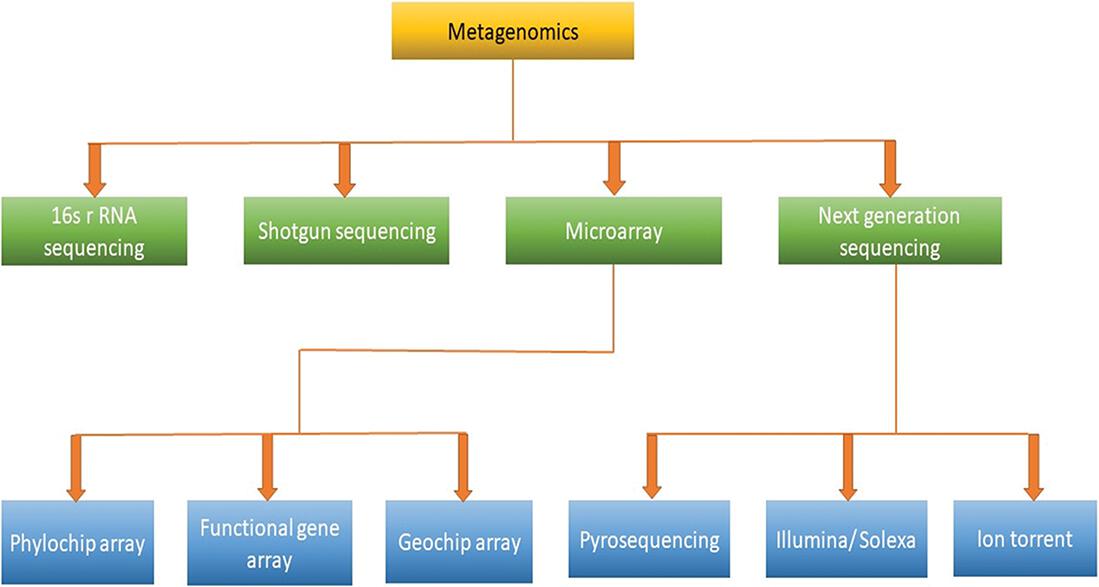
Figure 1.2: Overview of metagenomic approaches.
Few metagenomic techniques are discussed below.
1.2.1 16S rRNA
In all microorganisms, the identification of a highly conserved and variable 16S rRNA gene sequence is considered the standard for defining phylogenetic similarity between individuals in microbial communities [].
].
1.2.2 Shotgun sequencing
In microbial ecology, shotgun metagenomic sequencing is an effective method since it offers a robust and accurate assessment of microbial heterogeneity [].
1.2.3 Microarray
One of the most promising techniques in functional genomics is DNA microarray, which is commonly recognized as a DNA chip or a biochip. It is a collection of microscopic DNA spots that are deposited or synthesized by covalent or noncovalent interactions in a 2-D or 3-D array on a solid stationary surface such as silicon chips, glass, or nylon membrane. It enables the study, at once, of multiple genes without amplification of the individual genes by PCR [].
1.2.3.1 Phylochip array
It is a microarray of convenience and quality chips developed by the company Affymetrix to recognize various archaeal and bacterial organisms from intricate microbial populations. Without the need for any culture techniques, it provides a steadily fast, complete, and accurate testing method for samples taken from the environment. These chips contain extensive gene information and are broadly used in the recognition and study of mutations and polymorphisms depending on hybridization, such as single nucleotide polymorphisms or disease-relevant mutation evaluation. In severe biological systems such as effluents from industries, salters powered by the sun, coral reefs, and olive mill squanders, they have been used to examine microbial profiles [].










