Contents
List of Figures
List of Table
Guide
Pagebreaks of the print version

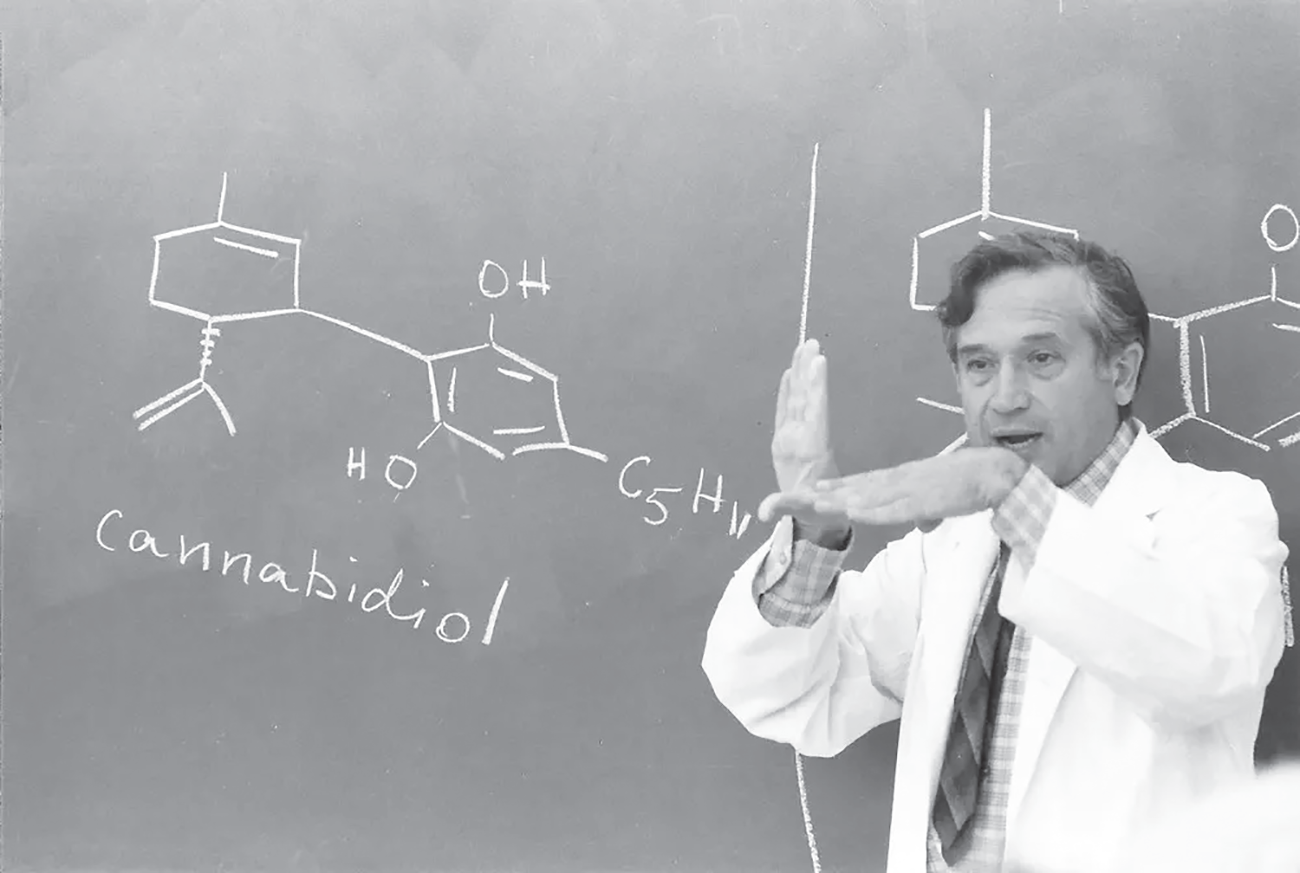
Raphael Mechoulam in a lecture circa 1964 explaining the structure of cannabidiol in comparison with that of 9-Tetrahydrocannabinol (on the blackboard behind him).
What Does the Science Say?
Linda A. Parker, Erin M. Rock, and Raphael Mechoulam
The MIT Press
Cambridge, Massachusetts|London, England
2022 Massachusetts Institute of Technology
This work is subject to a Creative Commons CC BY-NC-ND license.
Subject to such license, all rights are reserved.

The MIT Press would like to thank the anonymous peer reviewers who provided comments on drafts of this book. The generous work of academic experts is essential for establishing the authority and quality of our publications. We acknowledge with gratitude the contributions of these otherwise uncredited readers.
Library of Congress Cataloging-in-Publication Data
Names: Parker, Linda (Linda A.) author. | Rock, Erin M., author. | Mechoulam, Raphael, author.
Title: CBD : what does the science say? / Linda A. Parker, Erin M. Rock, and Raphael Mechoulam.
Description: Cambridge, Massachusetts : The MIT Press, [2022] | Includes bibliographical references and index.
Identifiers: LCCN 2021035141 | ISBN 9780262544054 (paperback)
Subjects: MESH: Cannabidiolpharmacology | Cannabidioltherapeutic use | Analgesicstherapeutic use | Neuroprotective Agentstherapeutic use | Anticonvulsantstherapeutic use
Classification: LCC RM666.C266 | NLM QV 95 | DDC 615.7/827--dc23
LC record available at https://lccn.loc.gov/2021035141
d_r0
Contents
- List of Figures
Articles published on CBD in the PubMed database over the years.
Schematic of FDA approval process
The synthesis of CBD
Reactions of CBD under acidic and basic conditions
Oxidations of CBD and CBD diacetate
Photochemical reactions of CBD
Reactions of cannabidiolic acid
CBD derivatives- List of Table
CBD pharmacodynamics
Preface
The writing of this book has been a labor of love for all three authors, having been drawn together by our keen interest in the science of cannabidiol (CBD). The first paper that Raphael Mechoulam published in the cannabinoid field (among nearly five hundred lifetime publications) was the identification of the structure of the CBD molecule (Mechoulam and Shvo 1963), which allowed its synthesis and an evaluation of its mechanism of action in several biological assays. For the past sixty years, several groups have collaborated with Mechoulam, conducting some of the earliest studies on the potential medicinal benefits of this compound. Among those collaborations was one with Linda Parkers laboratory in Canada, investigating the effects of cannabinoids on nausea, vomiting, anxiety, pain, and addiction in preclinical rodent models (see Mechoulam and Parker 2013). Erin Rock joined in this collaboration, first as an undergraduate at Wilfrid Laurier University (Waterloo, Ontario) and then as a graduate masters and PhD student at the University of Guelph, identifying the mechanism of action of the antinausea and antivomiting effects of CBD for her PhD research (Rock et al. 2012). Rock continued as a postdoctoral fellow/research associate with Parker, continuing to unlock the mysteries of CBD and several other cannabinoids using these models.
People have used the cannabis plant for millennia for its medicinal and mind-altering effects. This complex plant contains over 100 plant cannabinoids, including the most well known, 9-tetrahydrocannabinol (THC) and cannabidiol (CBD). Of the over 100 cannabinoid compounds in the cannabis plant, THC has been identified as essentially the only psychotropic compound, based on research by Raphael Mechoulams group in Israel and several others in the 1960s and 1970s. CBD, however, is not mind-altering.
Awareness of the potentially beneficial effects of CBD has grown at an astonishing rate in the mind of the general public, with Google Internet searches doubling in frequency every year for the past five years, and it is continuing to accelerate (Leas et al. 2019). Indeed, CBD has become a trendy ingredient in mass market products that make broad and at times unsubstantiated claims of its ability to treat a myriad of symptoms from skin disorders to chronic pain, as well as cosmetic useand in many cases, without human clinical trial evidence. Many pet owners are also administering CBD for management of conditions such as pain and anxiety without relevant scientific evidence for these indications.
This current CBD craze often generalizes to human health on the basis of findings in cells or in preclinical rodent research. However, human clinical trial research has severely lagged behind the basic cellular and preclinical animal research on the beneficial effects of CBD, with the exception of the use of CBD in rare forms of childhood epilepsy. The lack of clinical trial data is surprising, given that over sixty years ago, small-scale human trials for treatment of epilepsy, addiction, and anxiety showed that CBD may be a promising potential treatment option. However, the regulatory rules governing research with cannabis, a Schedule I drug, prohibited large-scale research with humans on the therapeutic potential of CBD. In recent years with countries such as Canada and several US states legalizing cannabis, one would expect that access to CBD has become much more feasible for large-scale human clinical trials. However, at the time of writing this book, this has not yet been the case. As consumers have increased access to a variety of cannabis products, US and Canadian scientists face the burden of strict regulatory scrutiny (Haney 2020) and have a limited variety of cannabis and CBD to evaluate in trials. Despite these barriers, a current survey of the National Institutes of Health website, www.clinicaltrials.gov , revealed 276 planned, ongoing, or completed human trials with CBD (the vast majority using oral formulations) for many of the indications that have shown promise in the preclinical research.
It must be emphasized that the standardized, chemically pure CBD available for preclinical research and human clinical trials is not necessarily the consumer CBD available for sale from vendors and the Internet. A 2017 survey (Bonn-Miller, Banks, and Sebree 2017) reported that of eighty-four online CBD and hemp oil products examined, only twenty-six were accurately labeled for CBD and THC content, with CBD often being overlabeled and THC underlabeled, consistent with warnings from the Food and Drug Administration. Buyer beware!
Many drugs used today are natural products or their derivatives. So far, CBD has been approved as a treatment by the US Food and Drug Administration only for some rare forms of childhood epilepsy and seizures associated with tuberous sclerosis complex in patients one year of age or older. In this book, we discuss various aspects of CBDs actions. In many disease states, mostly in animal models, but also some in human studies, positive results have been noted and published. In view of the encouraging animal as well as the limited human clinical data and the relatively low level of toxicity or major side effects, we expect that CBD or, more likely, CBD derivatives with an improved pharmacological profile may be developed as drugs to treat several additional medical conditions in the future.












