Page List

Published in 2009 by The Rosen Publishing Group, Inc.
29 East 21st Street, New York, NY 10010
Copyright 2009 by The Rosen Publishing Group, Inc.
First Edition
All rights reserved. No part of this book may be reproduced in any form without permission in writing from the publisher, except by a reviewer.
Library of Congress Cataloging-in-Publication Data
Roza, Greg.
Bromine / Greg Roza.
p. cm.(Understanding the elements of the periodic table)
Includes bibliographical references and index.
ISBN-13: 978-1-4358-5068-2 (library binding)
1. Bromine. 2. Bromine compounds. I. Title.
QD181.B7R69 2009
546.733dc22
2008014934
Manufactured in the United States of America
On the cover: Bromines square on the periodic table of elements. Inset: The atomic structure of bromine.
Contents
T he chemical industry today is an important and profitable business in the world economy, and it has been for a very long time. Thousands of years ago, countries exchanged goods and services along trade routes such as the Silk Road. Important trade items included precious gems and metals, spices, textiles, and dyes. Certain dyes became prized for their color and ability to last, especially when used to dye Chinese silks. Of these dyes, perhaps the most notable is Tyrian purple, which also became known as royal purple.
Tyrian purple dye was made from a fluid extracted from a few types of sea snails, called murex. The extraction process was long and difficult, which made the dye expensive. However, the dyes brilliant color made it a worthwhile expense for many wealthy nobles. Tyrian purple was sometimes as costly as precious metals, such as gold (Au) and silver (Ag). Many historians consider Tyrian purple dye and fabrics to be among the first products of the chemical industry.
It was not until many centuries later that people found out what gives Tyrian purple its brilliant colorthe element bromine (Br). Tyrian purple, a product of the ancient chemical industry, contains the element bromine. Today, bromine is an ingredient of many useful chemicals, including pesticides, dyes, disinfectants, and pharmaceuticals.

This is a mosaic from around the sixth century CE in the Basilica of San Vitale in Ravenna, Italy. It depicts Byzantine emperor Justinian I (center) wearing the Tyrian purple robes that many nobles of the time wore. Purple was a symbol of royalty and wealth.
Y ou might not be as familiar with the element bromine as you are with other elements, like copper (Cu) or oxygen (O). That is probably because the element bromine does not have many uses for the average person. For centuries, however, people have been using bromine in compounds. Compounds are substances containing the atoms of two or more elements joined together by chemical bonds.
The Royal Element
Compounds that contained bromine have been used since ancient times, specifically as fabric dye. These bromine compounds were first extracted from a tropical sea snail commonly known as the purple dye murex or the spiny dye murex. The scientific name of this murex is Haustellum brandaris. The ancient Greeks called this mollusk (shellfish) and the dyes made from it pophura, from which the modern word purple originated.
Various cultures around the world experimented with purple dyes that their peoples made from shellfish similar to the murex. Archaeologists have discovered shell mounds and dye pots in Qatar in the Middle East, in areas around the Mediterranean Sea, and in the Americas. Historians believe murex purple dyes were first used by the Minoan civilization of Crete around 1500 BCE. Dyed cloth was one of the Minoans most important trade products. In fact, many scientists believe murex purple dye is the oldest known pigment and perhaps the first chemical to be manufactured and traded on a wide scale.
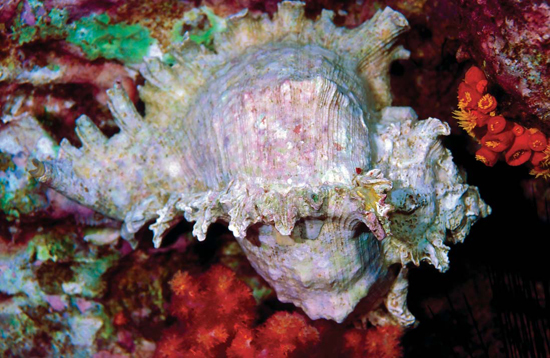
Shown here is a murex sea snail. Murex shells are often adorned with many long points or spines. A murex is sometimes called a spiny sea snail.
Around 1000 BCE, the ancient Phoenicians began to produce and trade cloth dyed with murex purple dye. The murex dye industry developed rapidly, thanks to the ancient Phoenicians in the city of Tyre (the site of the town Sur, Lebanon, today), and for this reason became widely known as Tyrian purple. Tyrian purple dye was both beautiful and long-lasting. Some fabrics dyed in Tyrian purple thousands of years ago still appear new and vibrant. The word Phoenicians may have derived from the Greek word phoinos, which means blood-red or purple-red.
Producing Tyrian dye was time-consuming and expensive. It became immensely popular among the upper classes of the ancient world. Roman nobles reserved the use of the purple dye for the robes of imperial family members, religious leaders, and other nobles. Even a single stripe of purple signified wealth and high social status. The color was occasionally called imperial purple or royal purple.
With the decline of the Roman Empire, the production of the dye decreased. The use of Tyrian purple diminished greatly with the fall of Constantinople in 1453. It was replaced by purple dyes that were easier and cheaper to make, like those made from lichens and madder, a Mexican cactus.
Whats in Tyrian Purple?
Scientists did not know that Tyrian purple dye contained bromine until the early 1900s. In 1909, German chemist Paul Friedlnder (18571923) obtained 1.4 grams (0.05 ounces) of pigment (the substance that produces color) from the glands of twelve thousand murices. After analyzing the sample, Friedlnder was surprised to find that it contained bromine. He discovered that the major component of murex dye is a chemical called 6,6-dibromoindigoa substance that had been synthesized in a laboratory in 1903. Scientific advances have greatly reduced the effort and time needed to synthesize 6,6-dibromoindigo, so it is no longer expensive.
Today, the term Tyrian purple is still used to describe a specific shade of purple. Tyrian purple dye extracted from snails is used in the analysis and restoration of ancient fabrics and paintings, but this natural dye is too time-consuming and expensive to make for widespread commercial applications.
Discovering Bromine
Bromine was discovered by two people independently at about the same time. In 1825, Carl Lwig (18031890), a German chemistry student at the University of Heidelberg, brought a sample of a brownish, bad-smelling liquid to his professor. He had prepared the sample by treating mineral water from a body of water near his home with chlorine (Cl2) gas. Lwig had planned to study the substance but put off the task in favor of other activities.
In 1826, French chemist Antoine Jrme Balard (18021876) identified the same substance as a new chemical element. Balard had obtained the substance from the waters of Montpellier salt marshes. He discovered that a layer of dark red liquid formed on top of the brine when chlorine gas was passed through it. Because the liquid had such a bad odor, Balard named the element bromine after

















