Published in 2010 by The Rosen Publishing Group, Inc.
29 East 21st Street, New York, NY 10010
Copyright 2010 by The Rosen Publishing Group, Inc.
First Edition
All rights reserved. No part of this book may be reproduced in any form without permission in writing from the publisher, except by a reviewer.
Library of Congress Cataloging-in-Publication Data
Roza, Greg.
The halogen elements: fluorine, chlorine, bromine, iodine, astatine /
Greg Roza.1st ed.
p. cm.(Understanding the elements of the periodic table)
Includes bibliographical references and index.
ISBN 978-1-4358-3556-6 (library binding)
1. HalogensPopular works. 2. Halogen compoundsPopular works.
3. Periodic lawPopular works. I. Title.
QD165.R69 2010
546'.73dc22
2009012539
Manufactured in the United States of America
On the cover: Illustrations of the halogen atoms, paired with each elements square from the periodic table. Clockwise from top left to right are chlorine, bromine, astatine, and iodine, with fluorine in the center.
Contents
Introduction
E verything in our world is made up of elementssubstances such as iron (Fe), gold (Au), oxygen (O), and tin (Sn). When combined, elements form more complex substances called compounds. All of the elements are listed on the periodic table, which allows students and scientists to easily observe how they are similar and how they are different.
The periodic table is made up of rows and columns of elements. The rows of elements are called periods, and the columns are called groups. The elements in a single period or group usually have common characteristics. At the same time, they can be very different.
Group 17 of the periodic table includes (from top to bottom) fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and astatine (At). These elements are called the halogens. They are the most reactive elements on the periodic table, meaning they quickly form compounds with other elements and are rarely, if ever, found isolated in nature. The pure forms of the halogens are all highly poisonous, but their compounds are often very useful. The halogens are also different in several ways. For example, the halogen group is the only one that contains all three states of matter (solids, liquids, and gases) at room temperature.
People have known of and used minerals that contain the halogens for thousands of years. Minerals containing fluorine have long been used to purify metals. Chlorine is a main component of salt, which was first used thousands of years ago to preserve and season foods. Ancient people in the Middle East used a special bromine compound to make an expensive and beautiful purple dye. Some halogens have even been used as currency! Read on to learn more about the amazing halogens.
Developed around 1846 by pioneer photographer John Plumbe, this is one of the first photographs of the U.S. Capitol inWashington, D.C.This type of photograph (called a daguerreotype) required the use of chemicals called silver halides, which contains one or more halogens.
Chapter One
Meet the Halogens
T his chapter will introduce you to each of the halogens, including their basic characteristics, how they were first used, and who discovered them and how. The halogens can be very dangerous because of their high reactivity. However, they are also used to make many useful substances.
Fluorine
Fluorine is a pale yellow gas that is highly corrosive and poisonous in its pure form. It is the most reactive element known to man, and it easily forms compounds with most elements. Fluorine reacts violently when combined with some elements, particularly hydrogen (H). At one time, liquid fluorine and liquid hydrogen were used as rocket fuel. Other fluorine compounds are used to make industrial acids, refrigeration chemicals, and insecticides. Fluorine is used in the enrichment of uranium (U), a highly radioactive element. Enriched uranium is used as fuel in nuclear reactors. A form of fluorine is also added to toothpaste and water supplies to help prevent cavities!
The mineral fluorsparcalcium fluoride (CaF2)has been used for thousands of years to help cleanse metals of impurities during smelting. Eventually, scientists began to suspect that fluorspar contained an unidentified element, but it was difficult to isolate. Today, we know thats because this element, fluorine, resists being separated from other elements.
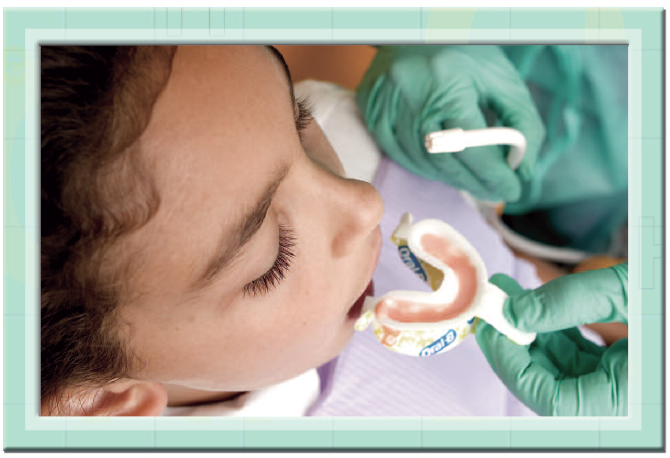
A dentist fits a mouthpiece filled with a fluoride treatment into a patients mouth. Much like the fluoride added to drinking water and toothpaste, this treatment is designed to strengthen the teeth and reduce the risk of cavities.
In 1886, French chemist Henri Moissan successfully isolated the element using a process called electrolysis on a solution of potassium hydrogen fluoride (KHF2) in liquid hydrogen fluoride (HF). Moissan earned the 1906 Nobel Prize in Chemistry for his discovery.
Chlorine
Chlorine is a pale green, strong-smelling gas. The element chlorine is very poisonous, but some of its compounds are very useful. Chlorine is used to make countless industrial and consumer products, including plastics, solvents, cleansers, dyes, and insecticides. An effective water purifier, it is commonly used to kill bacteria in drinking water and swimming pools. It is also used in many cleansers and disinfectants. Bleach is one of the most common products containing chlorine.
This woman is testing the chlorine levels in a public swimming pool. Chlorine is used to kill bacteria in pool water, and it is generally very safe. However, too much chlorine can irritate the skin and eyes.
The earliest known chlorine compound is sodium chloride (NaCl), also called halite or table salt. German-Swedish chemist Carl Wilhelm Scheele was the first person to isolate pure chlorine in 1774, although he wasnt aware of it. Scheele combined the mineral pyrolusite, or manganese dioxide (MnO2), with hydrochloric acid (HCl), creating a pale green gas. He thought the gas might contain oxygen. In 1810, English chemist Sir Humphry Davy established that the gas was a chemical element and named it chlorine.
What Is Electrolysis?
Electrolysis is the use of electricity to change one substance into another. This process can separate some elements from their compounds. Two electrodes are placed in a liquid that contains compounds of several elements, some of which are in the form of ions. Ions are electrically charged atoms or groups of atoms.The positive electrode (anode) attracts negatively charged ions in the liquid. The anode pulls the electrical charge off of the ion, which causes the ion to form a new substance. This new substance can be an element, such as a halogen. The negative electrode (cathode) attracts the positively charged ions.The cathode pushes charge into the ion, which also causes the ion to change into a new substance. These new substances build up on or around the electrodes until enough can be collected.This process is used to purify many elements, including fluorine and chlorine.