Contents
New York London
2014 Paul Parsons and Gail Dixon. All illustrations by Mark Franklin
All rights reserved. No part of this book may be reproduced in any form or by any electronic or mechanical means, including information storage and retrieval systems, without permission in writing from the publisher, except by reviewers, who may quote brief passages in a review. Scanning, uploading, and electronic distribution of this book or the facilitation of the same without the permission of the publisher is prohibited.
Please purchase only authorized electronic editions, and do not participate in or encourage electronic piracy of copyrighted materials. Your support of the authors rights is appreciated.
Any member of educational institutions wishing to photocopy part or all of the work for classroom use or anthology should send inquiries to Permissions c/o Quercus Publishing Inc., 31 West 57th Street, 6th Floor, New York, NY 10019, or to .
ISBN 978-1-62365-111-4
Distributed in the United States and Canada by Random House Publisher Services
c/o Random House, 1745 Broadway
New York, NY 10019
www.quercus.com
The Periodic Table
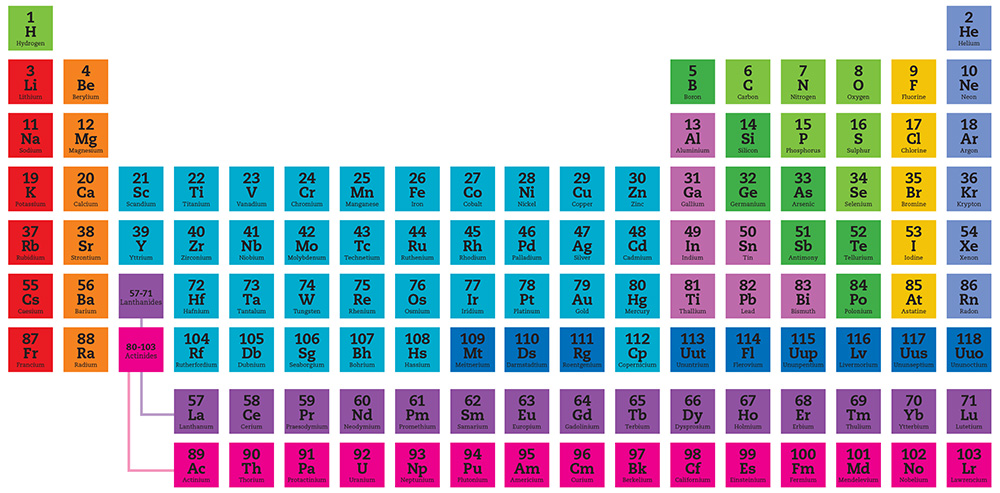
The periodic table shows the chemical elements ordered by atomic number (number of protons in the nucleus), but arranged in rows (periods) so that elements with similar chemistry occur in the same vertical column (group). Here, elements with the same chemical and physical properties are shown by the color categories identified in the key at right. In general, members of each category also have a similar chemical valency, a measure of the number of bonds an element can form. Each element is represented by its chemical symbol. Above the symbol is the elements atomic number and below is the elements name.
Element Categories
 Alkali metals
Alkali metals
 Alkaline earth metals
Alkaline earth metals
 Lanthanides
Lanthanides
 Actinides
Actinides
 Transition metals
Transition metals
 Post-transition metals
Post-transition metals
 Metalloids
Metalloids
 Other non-metals
Other non-metals
 Halogens
Halogens
 Noble gases
Noble gases
 Unknown chemical properties
Unknown chemical properties

Alkali metals
This group 1 of metals occupies the far-left column of the periodic table. They are all soft, but solid metals at room temperature, and are highly reactivefor example, when dropped in water.
Elements Include: .

Alkaline earth metals
The alkaline earth metals are silver-white metals at room temperature. The name is a term that refers to the naturally occurring oxides of these elements. For example, lime is the alkaline oxide of calcium.
Elements Include: .

Lanthanides
The lanthanide elements occupy a horizontal strip normally appended at the foot of the periodic table. Named after lanthanum, the first element in the series, they are generally found in less common mineral rocks, such as monazite and bastnasite.
Elements Include: .

Actinides
The actinides fill the second horizontal strip at the foot of the table. Named after their first element, actinium, they are all highly radioactive. So much so, that natural reserves of many of these elements have decayed away to nothing.
Elements Include: .

Transition metals
The transition metals occupy a broad swathe in the center of the periodic table. They are harder than the alkali metals, less reactive and are generally good conductors of both heat and electrical current.
Elements Include: .

Post-transition metals
Lying in a triangular region to the right of the transition metals, the post-transition metals are soft metals that mostly have low melting and boiling points. They also include mercury, which is liquid at room temperature.
Elements Include: .

Metalloids
The metalloid elements form a line between the metals and non-metals in the periodic table. Their electrical conductivity is intermediate between the two groups, leading to their use in semiconductor electronics.
Elements Include: .

Other non-metals
In addition to halogens and noble gases, there are other elements that are simply classified as other non-metals. They display a wide range of chemical properties and reactivities. They have high ionization energies and electronegativities, and are generally poor conductors of heat and electricity. Most non-metals have the ability to gain electrons easily. They have lower melting points, boiling points and densities than the metal elements.
Elements Include: .

Halogens
The halogens, known as group 17, are the only group to contain all three principal states of matter at room temperature: gas (fluorine and chlorine), liquid (bromine) and solid (iodine and astatine)all non-metals.
Elements Include: .

Noble gases
The noble gases are non-metals occupying group 18 of the table. They are all gaseous at room temperature and share the properties of being colorless, odorless and unreactive. Including neon, argon and xenon, they have applications in lighting and welding.
Elements Include: .













 Alkali metals
Alkali metals Alkaline earth metals
Alkaline earth metals Lanthanides
Lanthanides Actinides
Actinides Transition metals
Transition metals Post-transition metals
Post-transition metals Metalloids
Metalloids Other non-metals
Other non-metals Halogens
Halogens Noble gases
Noble gases Unknown chemical properties
Unknown chemical properties