THEODORE GRAY
The Elements
A Visual Exploration of Every Known Atom in the Universe
Photographs by Theodore Gray and Nick Mann
Table of Contents
Copyright 2009 by Theodore Gray
All rights reserved. No part of this book, either text or illustration, may be used or reproduced in any form without prior written permission from the publisher.
Published by
Black Dog & Leventhal Publishers, Inc.
151 West 19th Street
New York, NY 10011
Distributed by
Workman Publishing Company
225 Varick Street
New York, NY 10014
Cover and interior design by Matthew Riley Cokeley.
Simulated atomic emission spectra by Nino Cutic based on data from NIST.
Other physical properties data from Wolfram Mathematica; used with permission. All diagrams generated by Mathematica.
ISBN-13: 978-1-57912-895-1
eISBN: 978-1-60376-405-6
Library of Congress Cataloging-in-Publication Data available on file.
ALL PHOTOGRAPHS BY NICK MANN AND THEODORE GRAY EXCEPT AS FOLLOWS:
Berkeley Seal and TM 2001 UC Regents (top right).
About the Authors
Theodore Gray is the author of Theo Grays Mad Science: Experiments You Can Do At HomeBut Probably Shouldnt and Theodore Grays Elements Vault, as well as the author of Popular Science magazines Gray Matter column. He is cofounder of Wolfram Research, creators of the worlds leading technical software system, Mathematica and Wolfram|Alpha. With his company, Touch Press, Gray is the developer of bestselling iPad apps including The Elements, The Solar System, The Waste Land, and Skulls. He is the proprietor of periodictable.com and lives in Champaign-Urbana, Illinois.
Nick Mann is a freelance photographer. Aside from having photographed more elements than probably anyone in the world, he is an accomplished landscape, sports, and event photographer. He lives in Champaign-Urbana, Illinois.}
There is not anything which returns to nothing, but all things return dissolved into their elements.
Lucretius, De Rerum Natura, 50 BC
The periodic table is the universal catalog of everything you can drop on your foot. There are some things, such as light, love, logic, and time, that are not in the periodic table. But you cant drop any of those things on your foot.
The earth, this book, your footeverything tangibleis made of elements. Your foot is made mostly of oxygen, with quite a bit of carbon joining it, giving structure to the organic molecules that define you as an example of carbon-based life. (And if youre not a carbon-based life-form: Welcome to our planet! If you have a foot, please dont drop this book on it.)
Oxygen is a clear, colorless gas, yet it makes up three-fifths of the weight of your body. How can that be?
Elements have two faces: their pure state, and the range of chemical compounds they form when they combine with other elements. Oxygen in pure form is indeed a gas, but when it reacts with silicon they become together the strong silicate minerals that compose the majority of the earths crust. When oxygen combines with hydrogen and carbon, the result can be anything from water to carbon monoxide to sugar.
Oxygen atoms are still present in these compounds, no matter how unlike pure oxygen the substances may appear. And the oxygen atoms can always be extracted back out and returned to pure gaseous form.
But (short of nuclear disintegration) each oxygen atom can never itself be broken down or taken apart into something simpler. This property of indivisibility is what makes an element an element.
In this book I try to show you both faces of every element. First, you will see a great big photograph of the pure element (whenever that is physically possible). On the facing page you will see examples of the ways that element lives in the worldcompounds and applications that are especially characteristic of it.
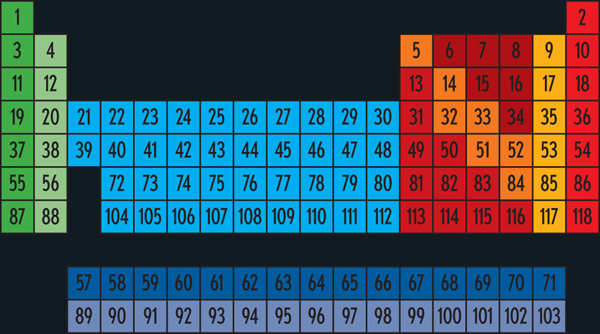
Before we get to the individual elements, its worth looking at the periodic table as a whole to see how it is put together.
The periodic table, this classic shape, is known the world over. As instantly recognizable as the Nike logo, the Taj Mahal, or Einsteins hair, the periodic table is one of our civilizations iconic images.
The basic structure of the periodic table is determined not by art or whim or chance, but by the fundamental and universal laws of quantum mechanics. A civilization of methane-breathing pod-beings might advertise their pod-shoes with a square logo, but their periodic table will have recognizably the same logical structure as ours.
Every element is defined by its atomic number, an integer from 1 to 118 (so farmore will no doubt be discovered in due time). An elements atomic number is the number of protons found in the nucleus of every atom of that element, which in turn determines how many electrons orbit around each of those nuclei. Its those electrons, particularly the outermost shell of them, that determine the chemical properties of the element. (Electron shells are described in more detail on )
The periodic table lists the elements in order by atomic number. The sequence skips across gaps in ways that might seem quite arbitrary, but that of course are not. The gaps are there so that each vertical column contains elements with the same number of outer-shell electrons.
And that explains the most important fact about the periodic table: Elements in the same column tend to have similar chemical properties.
Lets look at the major groups in the periodic table, as defined by the arrangement of columns.
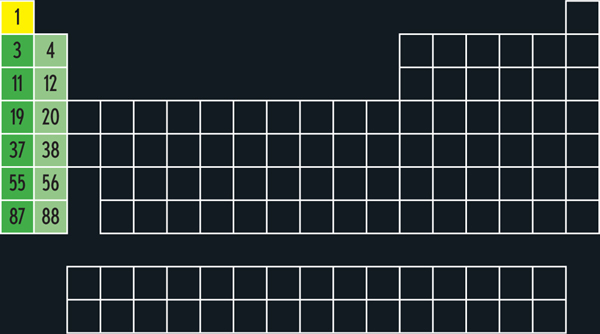
The very first element, hydrogen, is a bit of an anomaly. Its conventionally placed in the leftmost column, and it does share some chemical properties with the other elements in that column (principally the fact that in compounds, it normally loses one electron to form an H + ion, just as sodium, element 11, loses one electron to form Na + ). But hydrogen is a gas, while the other elements in the first column are soft metals. So some presentations of the periodic table isolate hydrogen in a category all its own.
The other elements of the first column, not counting hydrogen, are called the alkali metals, and they are all fun to throw into a lake. Alkali metals react with water to release hydrogen gas, which is highly flammable. When you throw a large enough lump of sodium into a lake, the result is a huge explosion a few seconds later. Depending on whether you took the right precautions, this is either a thrilling and beautiful experience or the end of your life as you have known it when molten sodium sprays into your eyes, permanently blinding you.
Chemistry is a bit like that: powerful enough to do great things in the world, but also dangerous enough to do terrible things just as easily. If you dont respect it, chemistry bites.
The elements of the second column are called the alkali earth metals. Like the alkali metals, these are relatively soft metals that react with water to liberate hydrogen gas. But where the alkali metals react explosively, the alkali earths are tamerthey react slowly enough that the hydrogen does not spontaneously ignite, allowing calcium (20), for example, to be used in portable hydrogen generators.
Next page