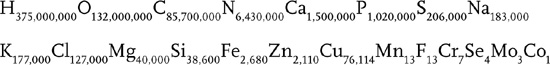Copyright 2019 by Tim James
Published in 2019 by Abrams Press, an imprint of ABRAMS. All Rights Reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted in any form or by any means, mechanical, electronic, photocopy, recording, or otherwise without permission in writing from the publisher.
Cataloging-in-Publication Data is available from the Library of Congress
ISBN: 978-1-4683-1702-2
Abrams books are available at special discounts when purchased in quantity for premiums and promotions as well as fundraising or educational use. Special editions can also be created to specification. For details, contact specialsales@abramsbooks.com or the address below.
Abrams Press is a registered trademark of Harry N. Abrams, Inc.

ABRAMS The Art of Books
195 Broadway, New York, NY 10007
abramsbooks.com
Dedicated to
the students of Northgate High School
Contents
INTRODUCTION
A Recipe for Reality
Fourteen billion years ago, our Universe decided to begin. We dont know what came before (if there was a before), we just know it started stretching in every direction and has been doing so ever since.
In the first few nanoseconds after the big bang, all of reality was a glowing soup of particles, frothing at temperatures millions of times hotter than the Sun. As everything spread out, however, things cooled, particles stabilized, and the elements were born.
Elements are the building blocks nature uses for cosmic cooking; the purest substances making up everything from beetroot to bicycles. Studying the elements and their uses is what we call chemistry, although sadly that word has come to mean something sinister for many people.
A writer on a popular health website was recently moaning about chemicals in our food and what we can do to keep food chemical free. These scaremongers seem to think that chemicals are toxins created by lunatics in lab coats, but this view is far too narrow. Chemicals arent just the bubbling liquids you see in test tubes: they are the test tubes themselves.
The clothes youre wearing, the air youre breathing, and the page youre currently reading are all chemicals. If you dont want chemicals in your food then Im afraid its too late. Food is chemicals.
Suppose you mix two parts of the element hydrogen with one part oxygen. In scientific notation, youd write that as H2O, water, the most famous chemical in the world. Chuck in a bit of the element carbon and you get C2H4O2household vinegar. Multiply each of those ingredients by three and youll get C6H12O6, more commonly known as sugar.
The only difference between cooking and chemistry is that while a recipe might specify a vegetable, chemistry wants to go deeper and find
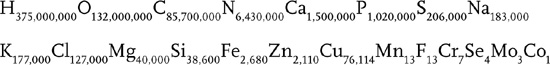
It looks like something you might find in a barrel of toxic waste but its the chemical formula for a human being. You have to multiply each number by seven hundred trillion, but those are the correct chemical ratios for one human body. So, if you hear someone say they distrust chemicals, feel free to reassure them. They are a chemical.
Chemistry is not an abstract subject happening in dingy laboratories: its happening everywhere around us and everywhere within us.
In order to understand chemistry, therefore, we have to understand the periodic table, that hideous thing you probably remember hanging on the wall of your chemistry classroom. Glaring down at you with all its boxes, letters, and numbers, the periodic table can be intimidating. But its nothing more than an ingredients list, and once youve learned to decode it, the periodic table becomes one of your greatest allies in explaining the Universe.
So, yes, the periodic table is seriously weird and seriously complicated, but so is the rest of nature. Thats what makes it worth studying. Thats what makes it beautiful.
CHAPTER ONE
Flame Chasers
THE MOST FLAMMABLE SUBSTANCE EVER MADE
Chemistry really began when we mastered our first reaction: setting fire to stuff. The ability to create and control fire helped us to hunt, cook, ward off predators, stay warm in winter, and manufacture primitive tools. Originally, we burned things like wood and fat, but it turns out that most substances are combustible.
Things catch alight because they come into contact with oxygen, one of the most reactive elements out there. The only reason things arent bursting into flame all the time is that while oxygen is reactive it needs energy to get going. Thats why starting a fire also requires something like warmth or friction. Oxygen has to be heated in order to combust.
The most flammable chemical ever made, though, far worse than oxygen, was created in 1930 by two scientists named Otto Ruff and Herbert Krug. Meet chlorine trifluoride.
Made from the elements chlorine and fluorine in a one-to-three ratio, chlorine trifluoride is unique in being able to ignite literally anything it touches, including flame retardants.
A green liquid at room temperature and a colorless gas when warmed, ClF3 will set fire to glass and sand. It will set fire to asbestos and Kevlar (the material from which firefighters suits are made). It will even set fire to water itself, spitting out fumes of hydrofluoric acid in the process.
There are very few instances of ClF3 being used, though, because it has the inconvenient property of setting fire to almost anything with which it comes into contact. It takes a special kind of maniac to think, Hmm, Ill give that a go.
The most spectacular ClF3 incident happened on an undisclosed
During the 1940s, a few cautious attempts were made to use it as a rocket fuel, but inevitably it kept setting fire to the rockets themselves so the projects were abandoned.
The only people who made a serious attempt to harness its power were the Nazi weapons researchers of Falkenhagen Bunker. The idea was to use it as a flame-thrower fuel, but it set fire to the flame-thrower and anyone carrying it so, again, it was deemed unusable.
Just think about that. Not only will it set fire to water, chlorine trifluoride is so evil even the Nazis didnt mess with it. What makes it so potent?
The answer is that fluorine behaves in a very similar way to oxygen but needs less energy to get started. Its the most reactive element on the periodic table and effectively out-oxygens oxygen at breaking other chemicals down. So, when you combine it with chlorine, the second most reactive element, you get an unholy alliance that starts fires without encouragement.
FIRE FROM WATER
The Greek philosopher Heraclitus was so enamored with fire he declared it to be the purest substancethe basic matter from which reality was made. According to him, everything was somehow made from fire in one form or another. Fire was, in other words, elemental.
Its an understandable assumption to make since fire does appear to possess magical properties. Then again, Heraclitus lived on a diet of nothing but grass and tried to cure himself of dropsy by lying in a cow shed for three days covered in manure after which he was So perhaps we dont need to take Heraclituss views too seriously.
The reason it was so difficult to identify elements in the ancient world was because, unknown to the early philosophers, very few elements occur in their pure state. Most of them are unstable and combine to form element fusions called compounds.