The periodic table
SINCE EARLY CLASSICAL TIMES, NATURAL PHILOSOPHERS (as the early scientists were known) had believed that all matter was comprised of an admixture of four basic elements earth, air, fire and water. This classification system persisted throughout the Middle Ages and into the Renaissance. However, over the course of the Enlightenment, thanks to the discoveries of the 18th-century French chemist Antoine Lavoisier, a new chemical classification emerged. Instead of four elements, there were now said to be 55 each of which had the quality of being fundamental, in that it was irreducible to any simpler substances.
Subsequently, chemists began trying to classify these elements according to their groups of properties, but with little success. Meanwhile, other spheres of science had already made spectacular achievements. Copernicus and Kepler had begun to explain the solar system, placing the sun at its centre. Newton had described the workings of this system by means of gravity. And Darwin had proposed how life itself had evolved according to natural selection. The discovery of a pattern amongst the chemical elements might well uncover a missing link between physics and chemistry: a key to life on earth and the behaviour of the universe at large. Some believed this might even provide a blueprint to the cosmos. This discovery occurred in 1869 with the classification of the chemical elements in a periodic table.
The man responsible for this breakthrough was the Russian chemist and inventor Dmitri Ivanovich Mendeleyev (18341907), born in a remote village near Tobolsk in Siberia, some 1,500 miles east of Moscow. He was the youngest of 14 or 17 children (no one seems quite sure, possibly owing to the number who did not survive childhood). His father had lost his teaching job when he went blind and had subsequently died when Dmitri was 13. This event had prompted Dmitris forceful mother Mariya to re-open the family glass-making factory, but within a year this enterprise collapsed when the factory burnt down. Recognizing young Dmitris outstanding academic talent, Mariya set off with him across Russia travelling by foot, by cart, on horseback, by riverboat and by train until finally they reached St Petersburg. Here she browbeat the authorities into letting the 16-year-old Mendeleyev study at the university, even though he had no relevant qualifications (Siberian educational certificates were not recognized in St Petersburg).
A year or so later, Mendeleyevs mother died and, overcome with grief and suffering from what would later be diagnosed as tuberculosis, her favourite son spent much of his remaining student life in bed. Illness and grief may have begotten physical idleness, but this did not extend to young Dmitris mental capacities. He was still able to indulge his seemingly insatiable appetite for knowledge, and he graduated in 1854 at the top of his year; two years later, he was further awarded a masters degree in chemistry.
Mendeleyev spent a couple of years working for Robert Bunsen at Heidelberg University, and in 1860 attended an international conference in Karlsruhe where significant time was devoted to the need to standardize chemistry, most notably by drawing up a classification of the elements. Mendeleyev returned to St Petersburg to teach in 1861 and was determined to improve the standard of chemistry teaching in Russia. In a typical frenzy of industry, he wrote a 500-page textbook in just 61 days. The result, Organic Chemistry, won him international recognition and the prestigious Demidov Prize (a sort of Russian precursor to the Nobel).
The periodic table is an arrangement of all of the chemical elements, ordering them in vertical columns according to their group (family) and across horizontal rows according to their periodic (regularly recurring) properties. Mendeleyevs fundamental insight states that, the elements, if arranged according to their atomic weights, exhibit an evident periodicity of properties.
By the exceptionally early age of 32 Mendeleyev had become a professor of chemistry at the University of St Petersburg, his salary enabling him to acquire a small country estate at Tver, 300 miles south of the city. Here he conducted many personal agricultural and scientific research projects, as well as proposing progressive ideas in fields ranging through mineral exploration, spectroscopy, the elimination of smoke from the funnels of Russian naval ships, and petrochemical refining. (According to his early opinion, the use of petroleum as fuel was akin to firing a kitchen stove with banknotes.) Despite being a polyglot he retained a lifelong aversion to what he described as high culture, such as literature and the arts. This would make him a difficult father-in-law when his daughter Lyubov married the great lyrical poet Alexander Blok in 1903.
Mendeleyevs early academic fame enabled him to travel widely, delivering lectures throughout Europe and in America. After visiting the oil fields of Pennsylvania he changed his mind about petrol, and on his return home he set about transforming the fledgling Russian oil fields by introducing revolutionary new refining techniques and laying gas pipelines.
Despite such cosmopolitanism, Mendeleyev retained and even exaggerated certain characteristics to reflect his Siberian origins. Previous generations of his family were said to have contained a number of Kirgiz Tartars, and he liked to play this to the hilt, claiming to have been brought up by Tartars in eastern Siberia and to have spoken no Russian until he was 17. In support of such fibs, there was no denying Mendeleyevs eccentric appearance, which became more exotic the older he got. He had a long, unkempt beard that ended in distinct points, the result of his obsessively combing it with his fingers when deep in thought. His head supported a large, shaggy halo of unkempt hair, which he is rumoured only to have cut on an annual basis: with the coming of spring, he would summon a local shepherd from his estate who would set about his masters hair with sheep shears. Little wonder that when Mendeleyev visited London the Scottish chemist William Ramsay described him as a peculiar foreigner, every hair of whose head acted in independence of every other.
A contemporary photograph shows Mendeleyev working at the desk of his study in St Petersburg. Behind him is a bookshelf with rows of bound volumes. Above his head, suspended from a hook on one of the shelves, hangs a key looking much like a kind of scientific halo, or exclamation mark (Eureka!) whilst, above the bookcase, high on the wall, hangs a row of framed portraits. Galileo, Descartes, Newton and Faraday Mendeleyevs scientific Gods gaze down at the wild-haired figure scribbling frantically amidst a disarray of papers scattered over his desk.
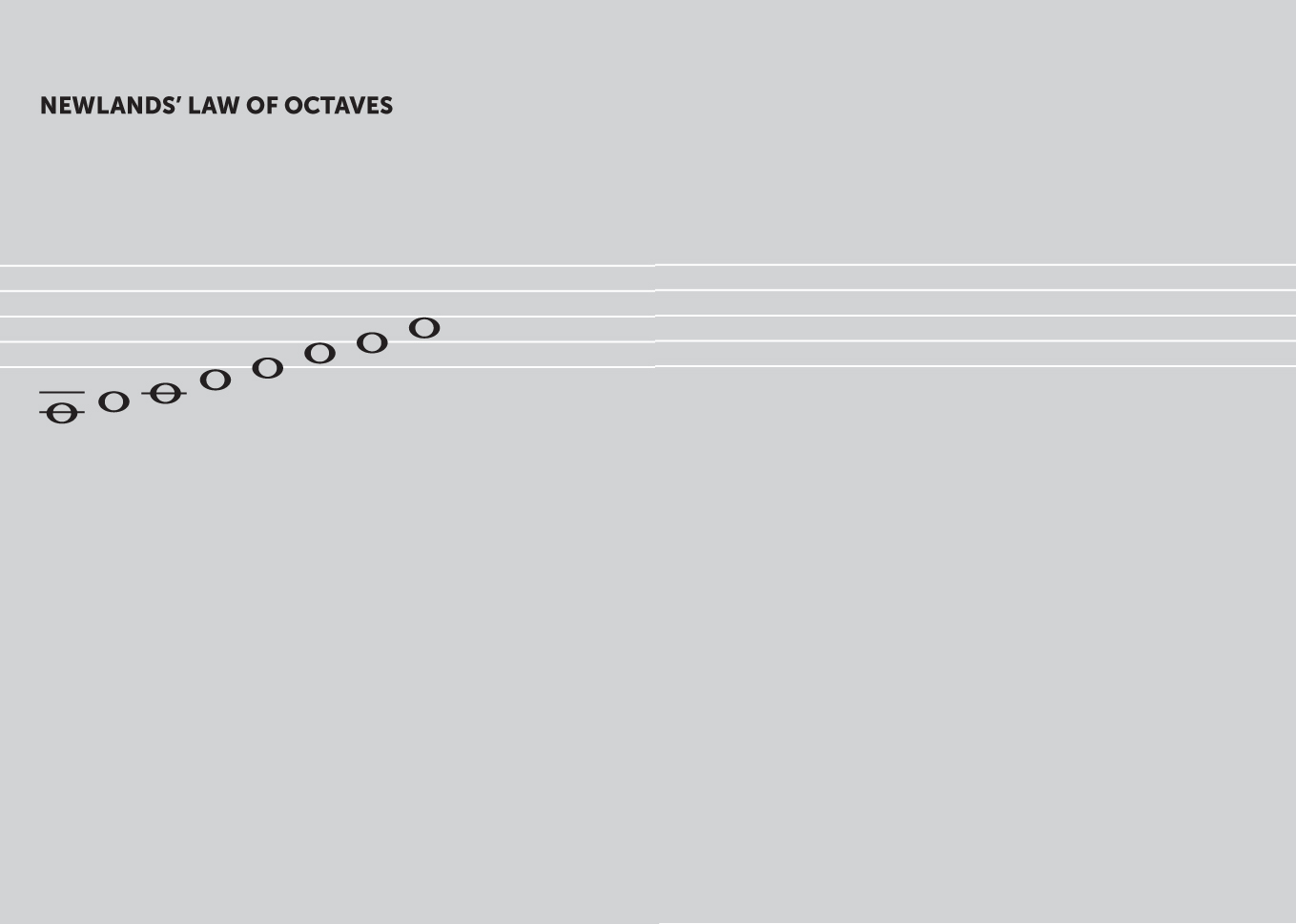
The English chemist John Alexander Reina Newlands (18371898) devised a periodic table of sorts some five years ahead of Mendeleyev, similar to the octaves in music. Newlands published his law of octaves in 1865, arranging elements by their atomic number into seven groups where, any given element will exhibit analogous behaviour to the eighth element following it in the table. Unlike the modern periodic table, periods were shown running from left to right and groups from top to bottom. However, the Society of Chemists declined to publish Newlands finding, and many contemporaries dismissed his theory.













