Contents
The Periodic Table
The periodic table shows the chemical elements ordered by atomic number (number of protons in the nucleus), but arranged in rows (periods) so that elements with similar chemistry occur in the same vertical column (group). Here, elements with the same chemical and physical properties are shown by the color categories identified in the key at right. In general, members of each category also have a similar chemical valency, a measure of the number of bonds an element can form. Each element is represented by its chemical symbol. Above the symbol is the elements atomic number and below is the elements name.
Element Categories
 Alkali metals
Alkali metals
 Alkaline earth metals
Alkaline earth metals
 Lanthanides
Lanthanides
 Actinides
Actinides
 Transition metals
Transition metals
 Post-transition metals
Post-transition metals
 Metalloids
Metalloids
 Other non-metals
Other non-metals
 Halogens
Halogens
 Noble gases
Noble gases
 Unknown chemical properties
Unknown chemical properties

Alkali metals
This group 1 of metals occupies the far-left column of the periodic table. They are all soft, but solid metals at room temperature, and are highly reactivefor example, when dropped in water.
Elements Include: .

Alkaline earth metals
The alkaline earth metals are silver-white metals at room temperature. The name is a term that refers to the naturally occurring oxides of these elements. For example, lime is the alkaline oxide of calcium.
Elements Include: .

Lanthanides
The lanthanide elements occupy a horizontal strip normally appended at the foot of the periodic table. Named after lanthanum, the first element in the series, they are generally found in less common mineral rocks, such as monazite and bastnasite.
Elements Include: .

Actinides
The actinides fill the second horizontal strip at the foot of the table. Named after their first element, actinium, they are all highly radioactive. So much so, that natural reserves of many of these elements have decayed away to nothing.
Elements Include: .

Transition metals
The transition metals occupy a broad swathe in the center of the periodic table. They are harder than the alkali metals, less reactive and are generally good conductors of both heat and electrical current.
Elements Include: .

Post-transition metals
Lying in a triangular region to the right of the transition metals, the post-transition metals are soft metals that mostly have low melting and boiling points. They also include mercury, which is liquid at room temperature.
Elements Include: .

Metalloids
The metalloid elements form a line between the metals and non-metals in the periodic table. Their electrical conductivity is intermediate between the two groups, leading to their use in semiconductor electronics.
Elements Include: .

Other non-metals
In addition to halogens and noble gases, there are other elements that are simply classified as other non-metals. They display a wide range of chemical properties and reactivities. They have high ionization energies and electronegativities, and are generally poor conductors of heat and electricity. Most non-metals have the ability to gain electrons easily. They have lower melting points, boiling points and densities than the metal elements.
Elements Include: .

Halogens
The halogens, known as group 17, are the only group to contain all three principal states of matter at room temperature: gas (fluorine and chlorine), liquid (bromine) and solid (iodine and astatine)all non-metals.
Elements Include: .

Noble gases
The noble gases are non-metals occupying group 18 of the table. They are all gaseous at room temperature and share the properties of being colorless, odorless and unreactive. Including neon, argon and xenon, they have applications in lighting and welding.
Elements Include: .

Unknown chemical properties
This is a label reserved for elements that can only be manufactured in a laboratory. Very often, only minute quantities of such elements have been createdmaking it impossible to ascertain their exact chemical classification.
Elements Include: .
Introduction
In the early 1860s, a Russian chemist working at St. Petersburg State University came up with an idea that would overturn our understanding of the chemical world. Dmitri Mendeleev put forward the idea of representing all of the known chemical elements in a table, according to their composition and properties. This insight not only enabled him to predict the properties of as-yet undiscovered elements, but would also shape the course of chemical research for evermore.
Mendeleev was fascinated by the chemical elements. Elements are the fundamental building blocks of chemistrychemical substances that can exist as individual atoms (as opposed to more complex chemical compounds, the smallest units of whichmoleculesare made by joining atoms of different elements together).
Mendeleev wondered if there was any way to order the elements according to their properties. He set about listing all the known elements (there were 62 of them at the time) by whats known as their atomic mass number. An atom consists of electrically neutral neutron particles and positively charged protons clustered tightly into a central nucleus, around which orbits a cloud of negatively charged electrons. The mass of the electron particles is so small compared to the others that its usually ignored. The neutrons and protons, however, are heavier; these particles weigh about the same as each other. Counting up the total number of neutrons and protons in an atoms nucleus yields a figure known as the atoms atomic mass number.












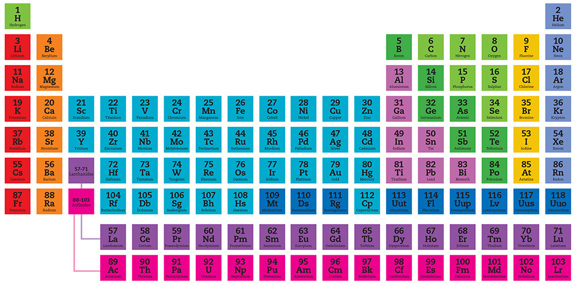
 Alkali metals
Alkali metals Alkaline earth metals
Alkaline earth metals Lanthanides
Lanthanides Actinides
Actinides Transition metals
Transition metals Post-transition metals
Post-transition metals Metalloids
Metalloids Other non-metals
Other non-metals Halogens
Halogens Noble gases
Noble gases Unknown chemical properties
Unknown chemical properties