
CONTENTS
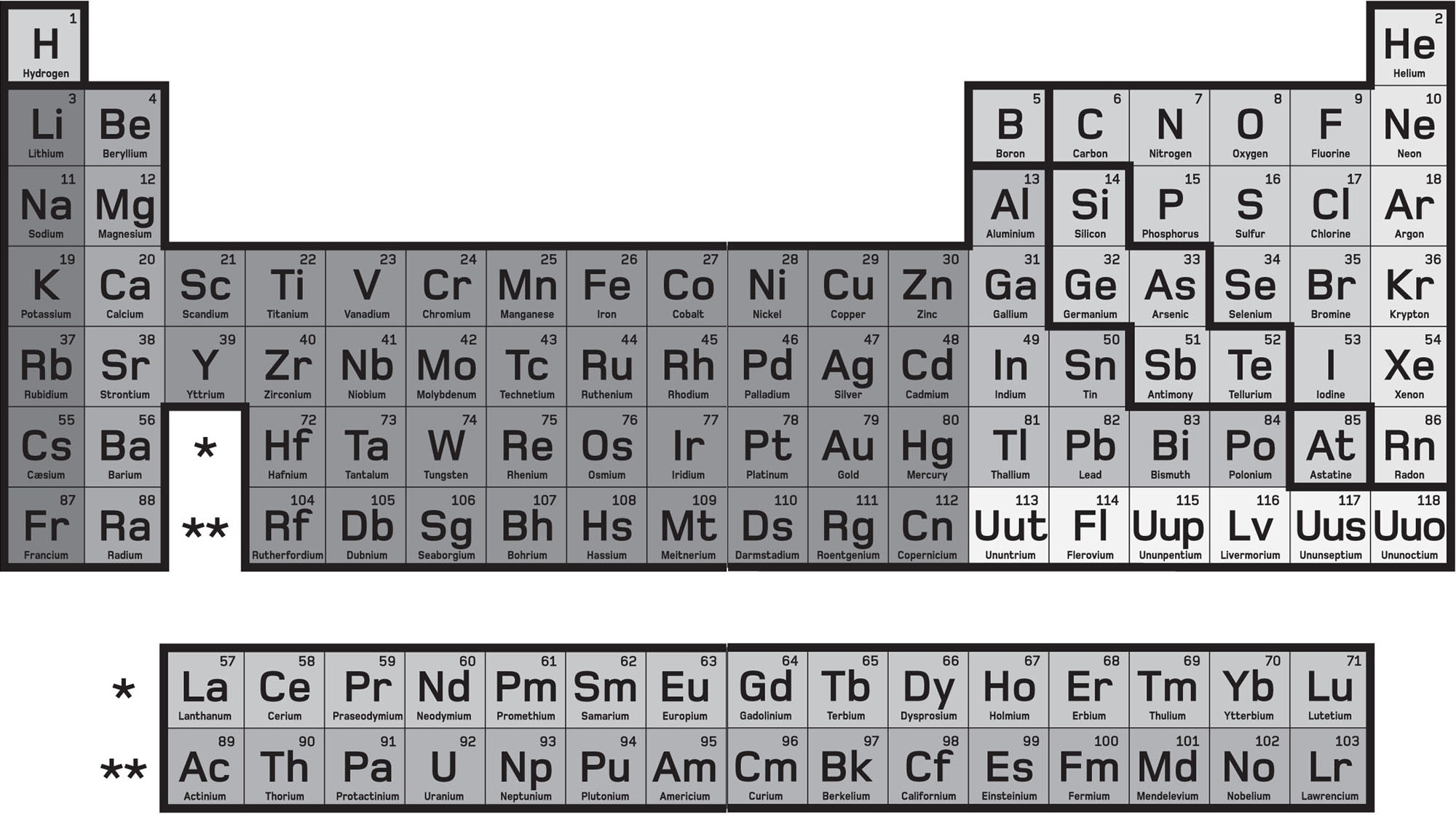
T he periodic table is one of the crown jewels of science. The classification of elements is one of the greatest and most highly prized discoveries, and is ranked in the first order of generalizations about our universe. The tables castle-like shape has become an instantly recognizable design icon a permanent (though not unchanging) feature on the wall of the chemistry lab, and on everything from mugs to novelty ties.
Russian chemist Dmitri Mendeleev invented the periodic table in 1869, using the chemical properties of the elements as an organizing principle. Its principal strength is that it was able to include and explain later findings, such as the internal make up of atoms, the discovery of atomic number and valence theories of chemical bonding. It even predicted the positions of several undiscovered elements. The turreted walls of the table encompass some of sciences most important discoveries, including atomic theory, electricity, the spectrum of light and other electromagnetic radiation, radioactivity and quantum physics.
The elements were once defined as the simplest substances of all the very word comes from the Latin word elementum , meaning first principle or most basic form. Today, we know that in fact every atom is composed of even smaller and more basic elementary particles, so a modern definition of an element might be a substance composed of atoms with the same number of subatomic proton particles in their nuclei. The investigation of the nature of matter and atomic structure has been a driving force in the history of science, leading not only to huge and sometimes surprising advances in our understanding of elements and atoms, but also to a wide range of technological applications that have shaped our modern society.
Like the law of gravitation or the theory of evolution, the periodic law, describing how chemical elements are ordered, is held to be universally true, and indeed, the properties of key biological elements formed part of the famous 1974 Arecibo message, humanitys first deliberate message to the stars. If we were ever to encounter an alien culture, this is the sort of common ground we could discuss: although our names for the elements would be different, we could all agree on the 118 different types of atom from which all matter is made.
T he Nobel-prize-winning physicist Richard Feynman was once asked what single scientific fact should be preserved in the event of an apocalypse. His response was definitive: that all things are made of atoms little particles that move around in perpetual motion, attracting each other when they are a little distance apart, but repelling upon being squeezed into one another. The atomic hypothesis is an ancient concept, dating back to the Ancient Greeks. However, evidence for small packages of matter comes from much later experiments in fields such as Brownian motion and atomic force microscopy (see ).
Elements are substances that cannot be broken down into simpler components, either by physical or chemical means. Each comprises only one variety of atom, determined by the number of protons in its nucleus (see ), 92 of which occur naturally on Earth.
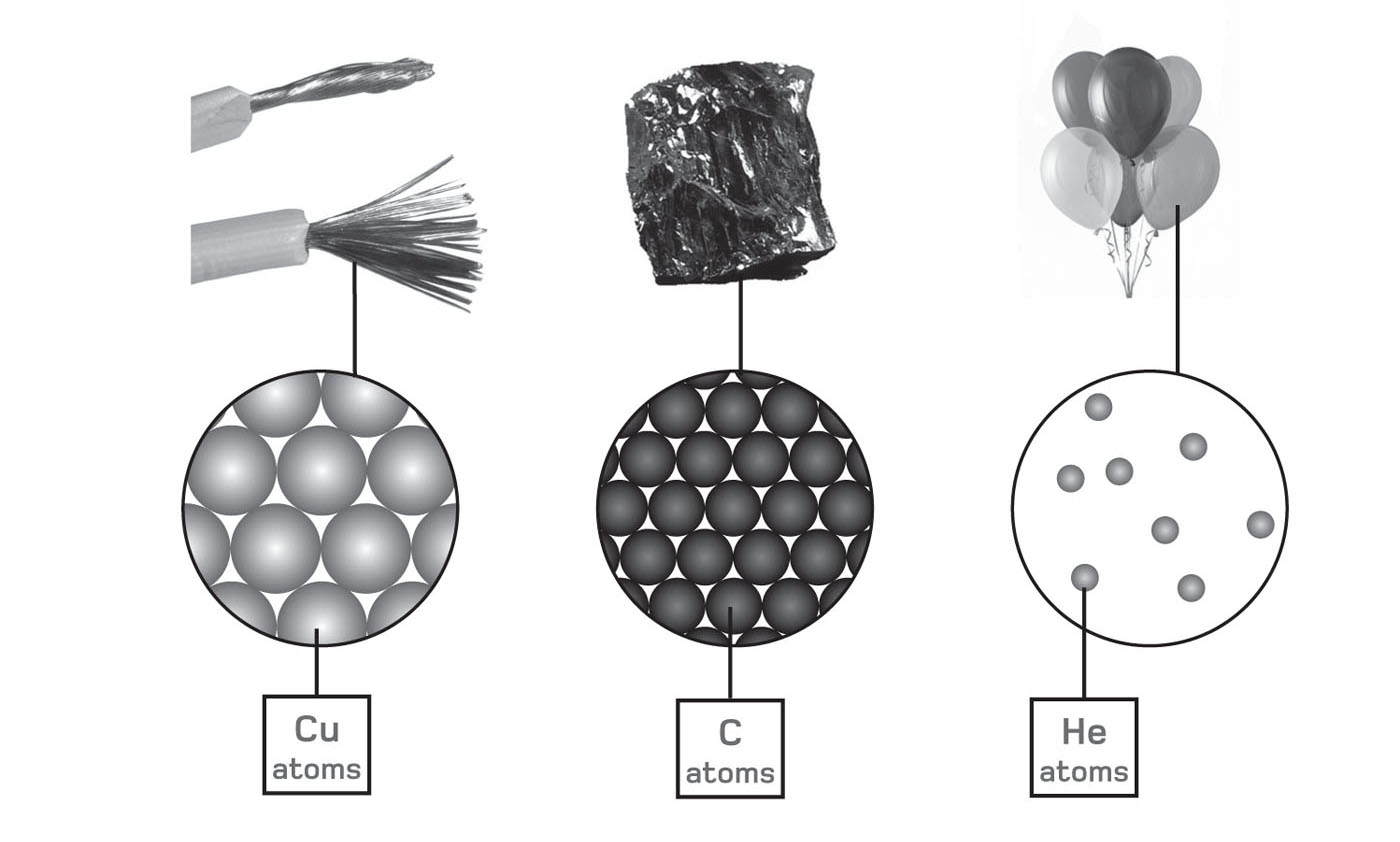
A pure element contains atoms of a single type for example the copper in electrical wire, the carbon in coal and the helium gas in party balloons. In practice, however, very few everyday materials are pure in this way.
Atomic structure
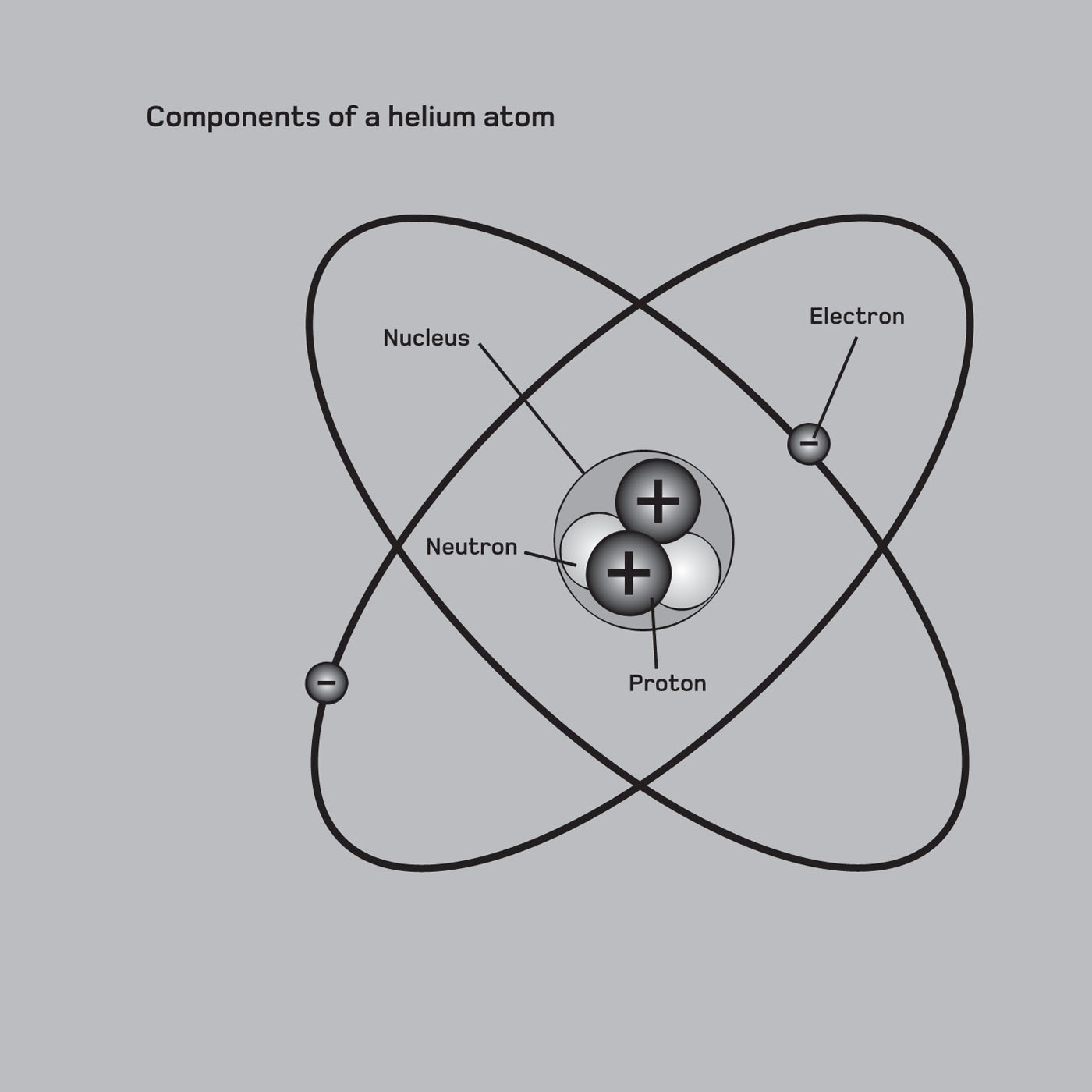
M ost people might have an idea of an atom as a mini solar system, the nucleus at the centre where the Sun would be, orbited by electron planets. The simplicity of this planetary model, sketched out by New Zealand physicist Ernest Rutherford in 1911 (see ).
The atomic nucleus consists of positively charged protons and electrically neutral neutrons, collectively called nucleons. Nearly all of the atoms mass is concentrated here. The number of protons determines the element even when they lose electrons, atoms still retain their identity. The charge of the protons is neutralized by an equal number of negatively charged electrons, bound by electrostatic attraction to the nucleus and resulting in an overall neutral atom. Atoms are typically about 1 ngstrom (0.1 nanometres) in diameter, about 100,000 times smaller than a red blood cell.
Subatomic particles
T he question of what is fundamental is a core pursuit of science, but the basic unit of matter differs depending on who is asked. For chemists, it is the atom, since this is how matter interacts in everyday conditions. For physicists, however, its the exotic elementary particles that spray out of high-energy proton collisions that lie at the heart of matter.
Atoms have three basic building blocks protons, neutrons and electrons. Of these, only the electron is thought to be fundamental. The electron was the first subatomic particle to be found, in 1897 (see ), and this was followed by discoveries of the proton in 1919 and the neutron in 1932. The electron belongs to the family of leptons, which also includes muon, tau and neutrino particles. Protons and neutrons are baryons composite particles made of different combinations of three quarks. These nucleons are heavy and almost all the intrinsic mass of an atom resides in the nucleus with them. Electrons are nearly 2,000 times lighter.
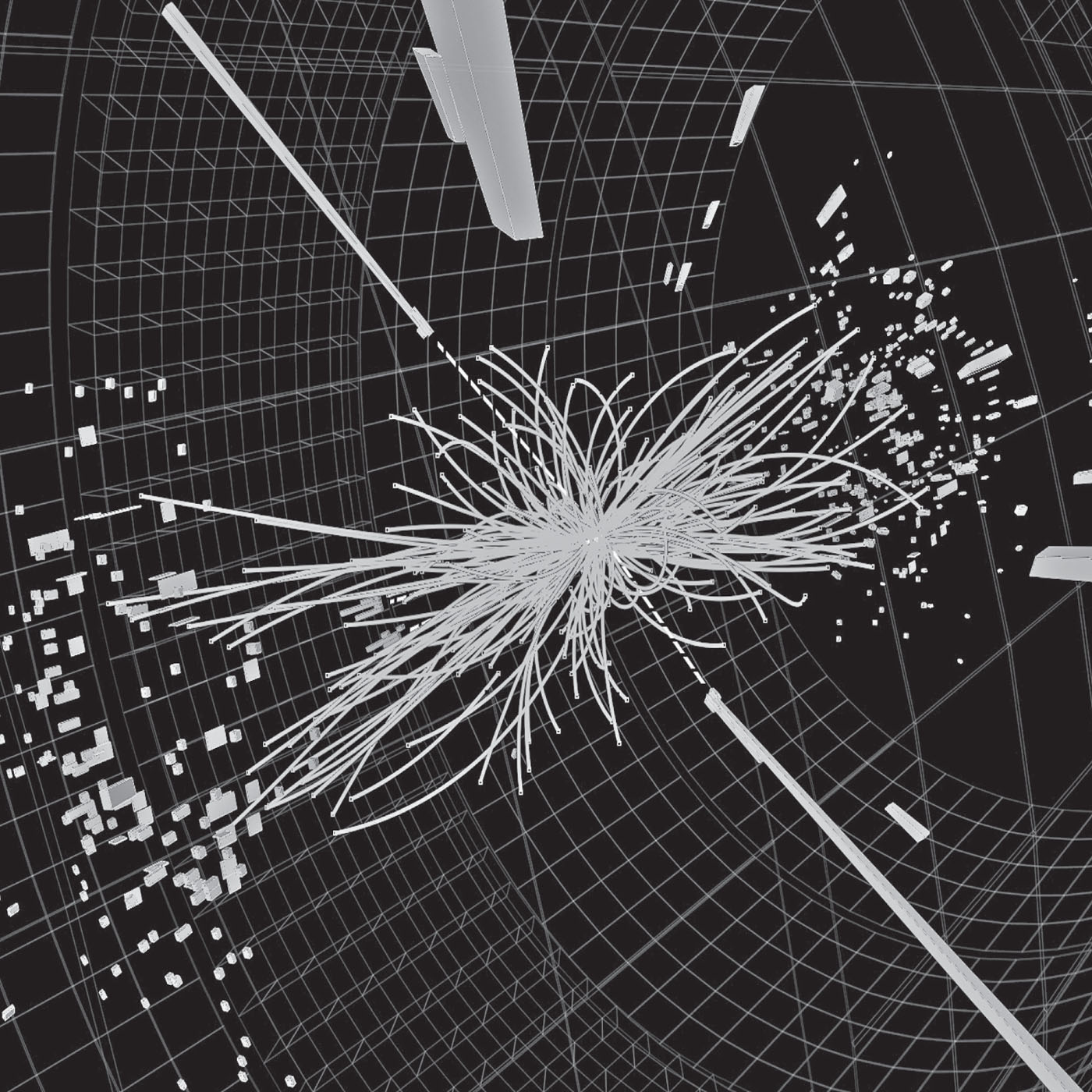
Tracks from subatomic particles in the Large Hadron Collider
Electron configuration
E lectrons in orbit around an atomic nucleus cannot take up any position they desire. Instead, they occupy atomic orbitals, arranged in shells that are associated with a fixed (quantized) energy. Each shell holds a specific number of electrons and when it is full, electrons start to fill up the next level. According to quantum physics, orbitals are not sharply defined structures, but are instead fuzzy-edged zones of probability, in which there is a 95 per cent probability of finding an electron. Gaps between orbitals are where electrons are never to be found.
An elements electron configuration is key to its physical properties and chemical behaviour. The number of electrons in the outermost shell (known as the valence shell) and their energies largely determine the type of bonding and range of compounds and element forms. Electrons in orbitals close to the nucleus are the most tightly bound, and a complete valence shell is a more stable electron configuration. This configuration also explains many of the trends observed in the periodic table (see ).
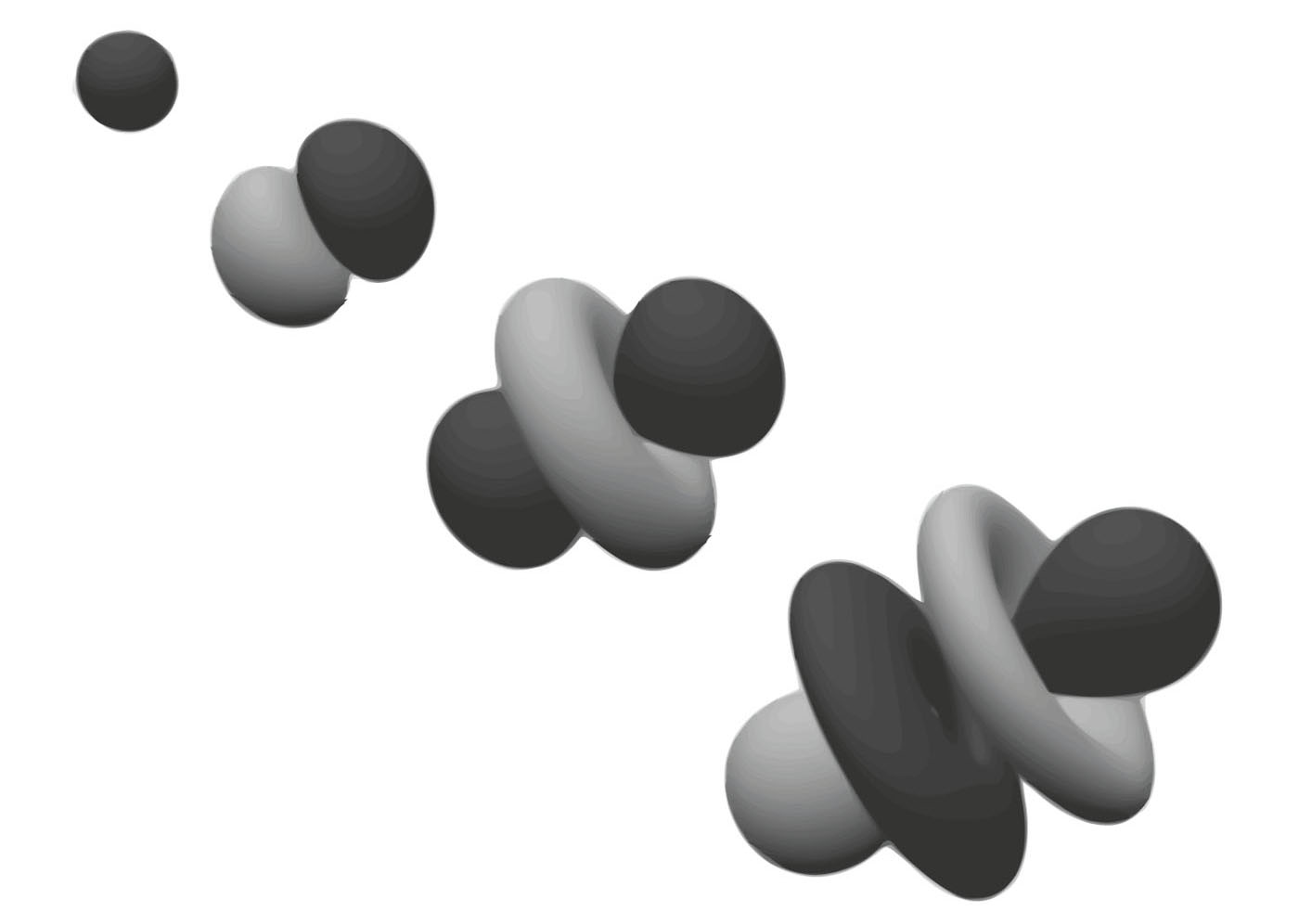
Electrons do not orbit the atomic nucleus like planets orbiting a sun. Instead, they smear out, forming an electron cloud around the nucleus. They are confined in limited spaces, called orbitals, arranged around the nucleus. The shapes of these orbitals are defined by mathematical functions.


















