

E-book published in 2012 by Encyclopdia Britannica, Inc., in association with Arcturus Publishing Limited, 26/27 Bickels Yard, 151-153 Bermondsey Street, London SE1 3HA. Britannica, Encyclopdia Britannica, and the Thistle logo are registered trademarks of Encyclopdia Britannica, Inc.
ISBN 978-1-61535-647-8 (e-book)
This edition first published in 2010 by Arcturus Publishing
Distributed by Black Rabbit Books
123 South Broad Street
Mankato
Minnesotta MN 56001
Copyright 2010 Arcturus Publishing Limited
All rights reserved
Planned and produced by Discovery Books Ltd.
www.discoverybooks.net
Managing editor: Laura Durman
Editors: Amy Bauman and Penny Worms
Designer: Ian Winton
Illustrator: Stefan Chabluk
Library of Congress Cataloging-in-Publication Data
Saunders, N. (Nigel)
Who invented the periodic table? / Nigel Saunders.
p. cm. -- (Breakthroughs in science and technology)
Includes index.
Summary: Looking at some of the major inventions and discoveries shaping our world today, Breakthroughs in Science profiles the research leading up to the discovery (not just profiles of the one or two key players). Each book describes the famous moment and then examines the continued evolution illustrating its impact today and for the future Provided by publisher.
1. Periodic lawTablesJuvenile literature. 2. Mendeleyev, Dmitry Ivanovich, 1834-1907--Juvenile literature. 3. Discoveries in scienceJuvenile literature. I. Title.
QD467.S325 2011
546.8dc22
2010011016
Picture Credits
Corbis: cover (Steve Allen/Brand X), 13 (Stefano Bianchetti), 16 (Bettmann), 35 (Bettmann), 36, 38 top (Hulton-Deutsch Collection),
40 (Peter Ginter/Science Faction).
Getty Images: 21 (SSPL).
IBM Corporation: 30.
iStockphoto: 14, 23 bottom (Mladen Mladenov).
NASA: 42/43.
Picture the Past: 10 (Image Courtesy of Derby Museums and Art Gallery and www.picturethepast.org.uk).
Science Photo Library: title page and 19 (Claude Nuridsany & Marie Perennou), 12, 15 (Sheila Terry), 23 top (Charles D. Winters), 24 left, 27, 37 (ISM), 38 bottom (Los Almos National Laboratory), 41.
Shutterstock: 7 (Monkey Business), 11 (Mark William Penny), 18 (Alexander Lason), 29 (Stefan Glebowski), 31 (Roman Sigaev), 32 (HomeStudio), 33, 34 (C).
SL001446US
Supplier 03, Date 0510
The IUPAC has recently recommended alternative spellings for the names of some elements and groups of elements, for example sulfur and actinoids. For clarity, we have decided to use traditional spellings in this book.
Chemical elements
The periodic table
The periodic table is a very useful invention for chemists, the scientists who study the substances around us. It is a chart containing all the known chemical elements. It even has spaces for elements not yet discovered. To understand the periodic table, how it was invented, and why it is needed by chemists, it is important to know about atoms and elements.
Atoms
Books, birds, buildings and all the other things around you are made from tiny things called atoms. You are made from atoms, too. Atoms are so small that a million of the biggest ones would make a pile less than half a millimeter high. We cannot see atoms, even with a microscope. It can be difficult to believe that something invisible exists. Just over a hundred years ago, a few scientists still did not believe in atoms, but the results of many experiments show that atoms are real. Not only that, they are made from even smaller things called subatomic particles.
Inside an atom
There are three main types of subatomic particle. Protons and neutrons are the heaviest. They join together to make a cluster in the middle of the atom, called the nucleus. Electrons are much lighter subatomic particles, and clouds of them surround the nucleus.
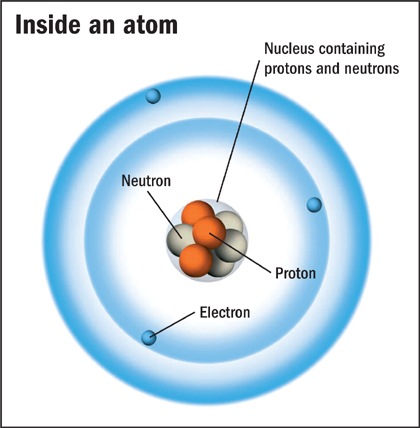
This is a diagram of a lithium atom. Its nucleus is surrounded by three electrons, and it contains three protons and four neutrons.
T HATS A F ACT !
The nucleus of an atom is around 100,000 times smaller than the atom as a whole, and most of an atom is empty space. The name atom comes from the Greek word atomos, meaning something that cannot be split.

Everything is made from atoms, even you. Oxygen atoms make up 65 percent of your body, and carbon atoms make up 18 percent of it. Hydrogen atoms make up most of the rest, along with small percentages of many other elements.
Elements
A chemical element is a substance made from one type of atom. Some elements, such as gold and iron, have been known for thousands of years. Others have been discovered much more recently. Helium, often used in balloons, was discovered in 1895. Americium, used in smoke alarms, was discovered only in 1944. Scientists continue to discover new elements today.
The atomic number (proton number) of an atom is the number of protons it contains. All the atoms of an element have the same atomic number. Hydrogen and carbon are two different elements. Every hydrogen atom contains just one proton, but every carbon atom contains six protons. There are over a hundred different elements, and no two elements have the same atomic number. The periodic table helps chemists understand all the different elements and what they do.
The periodic table of elements
A closer look
The periodic table might seem confusing at first, so lets look at its main features. Each box contains information about a single element, such as hydrogen or oxygen. You will not find water or any other compound in the periodic table. This is because compounds are made from two or more elements chemically joined together. For example, water is a compound made up of two hydrogen atoms and one oxygen atom.
Chemical symbols
Each element has its own chemical symbol, made from one, two, or sometimes three letters. The first letter is always a capital letter, and any others are lower case. Some symbols are easy to understand, such as H for hydrogen and C for carbon. Others, such as Cu for copper, are not so obvious.
T HATS A F ACT !
The ancient Romans had copper mines on Cyprus, an island in the Mediterranean Sea. They called the metal aes Cyprium, which became shortened to cuprum. That is why the symbol for copper is Cu and not Co (which is the symbol for cobalt).
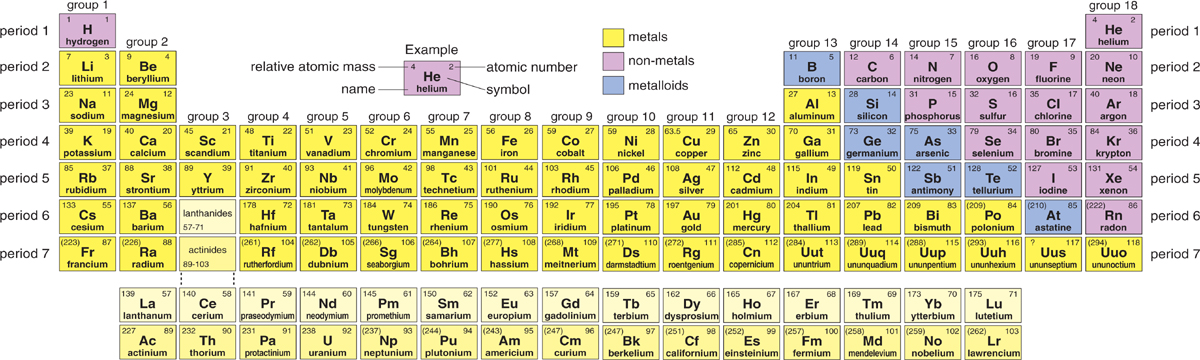
The periodic table of elements. The number on the left in each box is the elements relative atomic mass. The number on the right is its atomic number (proton number).
The numbers
Atoms are not only very small, they are also very light. They are so light that it makes little sense to use their actual masses in grams. Instead, their
Next page













