Top Drugs
History, Pharmacology, and Syntheses
Jie Jack Li
Oxford University Press is a department of the University of Oxford. It furthers the Universitys objective of excellence in research, scholarship, and education by publishing worldwide.
Oxford New York Auckland Cape Town Dar es Salaam Hong Kong Karachi Kuala Lumpur Madrid Melbourne Mexico City Nairobi New Delhi Shanghai Taipei Toronto
With offices in Argentina Austria Brazil Chile Czech Republic France Greece Guatemala Hungary Italy Japan Poland Portugal Singapore South Korea Switzerland Thailand Turkey Ukraine Vietnam
Oxford is a registered trade mark of Oxford University Press in the UK and certain other countries.
Published in the United States of America by Oxford University Press 198 Madison Avenue, New York, NY 10016
Oxford University Press 2015
All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted, in any form or by any means, without the prior permission in writing of Oxford University Press, or as expressly permitted by law, by license, or under terms agreed with the appropriate reproduction rights organization. Inquiries concerning reproduction outside the scope of the above should be sent to the Rights Department, Oxford University Press, at the address above.
You must not circulate this work in any other form and you must impose this same condition on any acquirer.
Library of Congress Cataloging-in-Publication Data
Li, Jie Jack.
Top drugs : history, pharmacology, syntheses / Jie Jack Li.
pages cm
Includes bibliographical references and index.
ISBN 9780199362585 (hardback)
ebook ISBN 9780199362608
1. DrugsHistory. 2. DrugsDesign. 3. Pharmaceutical chemistry.
I. Title.
RS420.L535 2015
615.1dc23 2015016478
9 8 7 6 5 4 3 2 1
Printed in the United States of America on acid-free paper
To Prof. Tami Spector
Contents
This book originated from a short course that I taught at the University of Pittsburgh. My course on the history, pharmacology, and synthesis of drugs has been warmly received by graduate students and senior undergraduate students majoring in chemistry, biology, pre-med, pharmacy, and related topics. Several professors who teach undergraduate organic chemistry asked me to develop a short textbook that will acclimate undergraduates to the real world of chemistry and drug discovery.
This book is geared toward undergraduate institutions interested in offering a short course on this topic. Senior undergraduate students and scientists in both academia and industry will also find it useful to understanding the landscape of current drug discovery and development.
This book covers the history, pharmacology, and synthesis of ten top drugs. Each chapter is divided to the following sections:
History
Pharmacology
2.1 Mechanism of Action
2.2 StructureActivity Relationship
2.3 Bioavailability, Metabolism, and Toxicology
Synthesis
3.1 Discovery Route
3.2 Process Route
Concluding Remarks
References
I am very much indebted to Prof. Neil K. Garg and Dr. Travis C. McMahon at UCLA for proofreading portions of the final version of the manuscript. Their knowledge and input have tremendously enhanced the quality of this book. Any remaining errors are, of course, solely my own responsibility.
As always, I welcome your critique! Please send your comments to this email address: lijiejackli@gmail.com.

Jie Jack Li
August 2014
San Francisco, California
Top Drugs
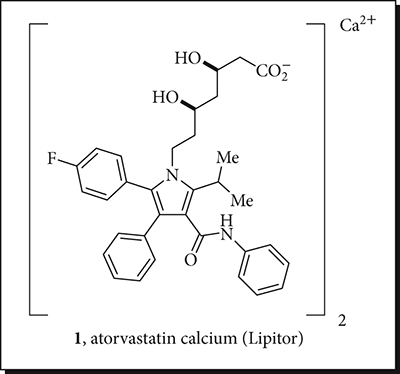
| USAN: | Atorvastatin Calcium |
| Brand Name: | Lipitor (Pfizer) |
| Molecular Weight: | 1155.37 (Parent 557.25) |
| FDA Approval: | 1996 |
| Drug Class: | Statins |
| Indications: | Lower Blood Cholesterol in Low-Density Lipoprotein (LDL) |
| Mechanism of Action: | Inhibitor of HMG-CoA Reductase |
To the human body, cholesterol () is a Janus-faced molecule. On the one hand, it is an indispensable building block for lifeabout 23% of total body cholesterol resides in the brain, making up one-tenth of the solid substance of the brain. Red blood cell membranes are also rich in cholesterol, which helps stabilize the cell membranes and protect cells. Furthermore, cholesterol is also the precursor of hormones such as progesterone, testosterone, estrogen, and cortisol. On the other hand, cholesterol helps plaque buildup, which constricts or blocks arteries, leading to angina, heart attack, stroke, and many other cardiovascular diseases. To date, the experimental, genetic, and epidemiologic evidence all point to escalating cholesterol levels as a major risk factor for cardiovascular diseases. Other major risk factors include obesity, diabetes, hypertension, smoking, and inactive lifestyle.
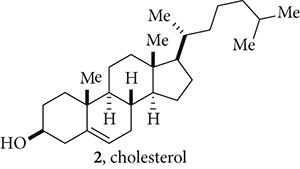
Depending on different water-soluble carriers, cholesterol could have starkly opposing effects on the heart. Cholesterol in low-density lipoprotein (LDL), often known as bad cholesterol, is the fundamental carrier of blood cholesterol to body cells. It can slowly build up in the walls of the arteries feeding the brain and heart and can form plaques. In contrast, cholesterol in high-density lipoprotein (HDL), frequently dubbed good cholesterol, is a carrier that takes cholesterol away from the arteries and brings it to the liver, where it can be removed from circulation by metabolism. The higher the levels of HDL, the better. In general, women have higher levels of HDL, which may explain why women have longer life expectations than men. Their higher levels of estrogen are somehow correlated to higher HDL-cholesterol levels.
Many attempts have been made to lower cholesterol levels. In the 1950s and 1960s, estrogen was tried but was quickly abandoned because it caused feminizing side effects on men. Thyroid hormone also had unacceptable side effects, such as trembling. Resins such as cholestyramine were used as bile acid sequestrants, or bile acid binding resins. The approach was not popular in patients because they were difficult to swallowliterally. One of the early cholesterol-lowering drugs still in use today is nicotinic acid (), which has been available since 1955. The advantage of nicotinic acid is that it also boosts the levels of HDL cholesterol. The disadvantage of nicotinic acid is that it often causes flushing as a side effect.
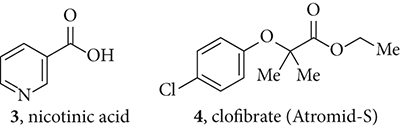
In 1954, Imperial Chemical Industries (ICI) discovered that clofibrate (, Atromid-S) possessed significant cholesterol-lowering activity and marketed it in 1958. Parke-Daviss gemfibrozil (, Lopid), launched in 1982, was the second fibrate on the market. In order to find safer analogs of clofibrate (), Parke-Davis screened over 8000 compounds similar to clofibrate using animals. Abbotts fenofibrate (, Tricor), is also a clofibrate analog. Fibrates have been found to be