A scientific guide to protein intake with training
What to eat, how much and when
Gommaar D'Hulst & Henning Langer
Health is the greatest of human blessings
- Hippocrates -
W ho are we? A quick look into the background of the authors.
Gommaar DHulst, PhD
Gommaar studied Sport Sciences and Exercise Physiology at the University of Leuven in Belgium where he also obtained his PhD. During his PhD he studied the molecular biology of the working skeletal muscle. Currently he is working at the state-of-the-art Laboratory of Exercise and Health at the ETH Zurich where his research focuses on topics like muscle health and muscle memory. Gommaar has always been very passionate about communicating evidence-based information to the public. In his spare time you can find him playing with his 2-year-old son or at CrossFit Kreis9 for his workout of the day.
Henning Langer, PhD (c)
Henning is a Postdoctoral Researcher in muscle physiology at the University of California, Davis. His work investigates skeletal muscle health and how it can be modulated through exercise and nutrition. He did his PhD at the Charit - Universittsmedizin Berlin, focusing on changes of muscle metabolism in neuromuscular disorders. Previously, he obtained his MSc from Maastricht University for work on skeletal muscle protein turnover in response to protein ingestion and resistance exercise. Henning is a certified strength and conditioning and CrossFit Level-1 coach. He has competed as an individual and as part of a team in CrossFit European Throwdowns and Sanctionals, including the German- and the French Throwdown.
Table of contents
Background
1.1. Protein: the building blocks
1.2. Muscle protein synthesis
1.3. Muscle protein breakdown
1.4. Net protein balance and changes in muscle size
1.5. Why measure muscle protein turnover and not just muscle growth?
Protein consumption to optimize adaptations to strength training
2.1. How much protein after strength training works best?
2.2. 20 gram of protein: one-size-fits-all?
2.3. Is there a difference between exercising one muscle group compared to the whole body when it comes to protein intake?
2.4. Protein: how much per day?
Protein quality: are all protein sources the same?
3.1. What determines protein quality?
3.2. Leucine - why is it so important? An introduction to the cell biology of MPS
3.3. Is leucine alone enough to maximize mTOR, MPS and growth?
3.4. Leucine availability in foods and its effect on total protein intake: Implications for plant-based proteins
3.5. What about other macronutrients? Do carbohydrates and fat augment muscle protein synthesis?
Protein timing
4.1. The anabolic window of opportunity
Protein Distribution
Protein before bed
Protein and ageing
Protein and weight loss
Protein and endurance exercise
Conclusion
Closing Remarks
. Acknowledgements
1. Background
1.1. Protein: the building blocks
By Gommaar DHulst
You have heard some version of this a lot: protein is essential to optimally recover from a hard workout, or protein helps to build muscle mass during periods of strength training and proteins are the building blocks for your muscles. But why is protein so important?
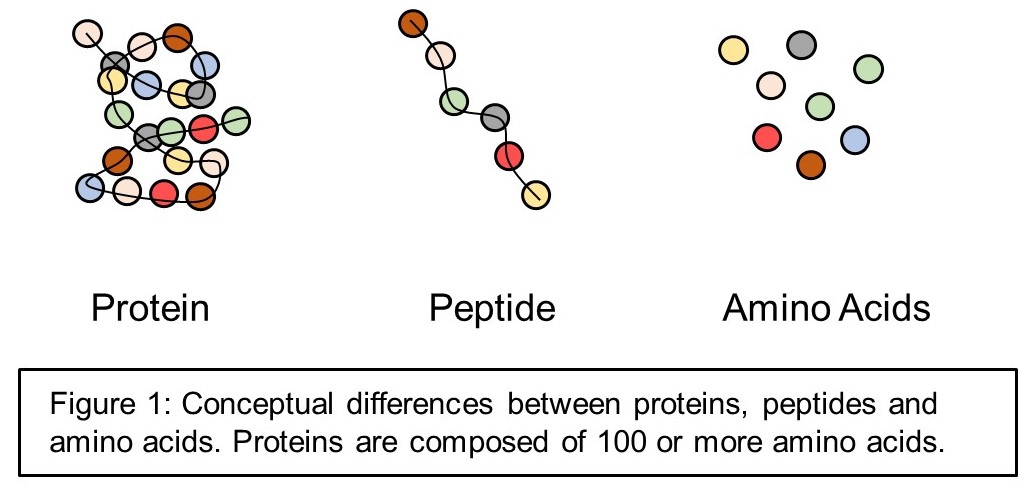
The proteins you eat are broken down into little chains of amino acids (peptides), which are eventually further broken down into single amino acids.
S keletal muscle accounts for approximately 40% of the body weight in humans, making it the largest organ by weight (skin covers more surface area, but weighs less). Healthy skeletal muscle is comprised of approximately 20% protein, making protein its main structural, functional and biochemical component. As protein itself is comprised of amino acids, it could be said that amino acids are the primary building blocks of the human body. Indeed, the name itself already suggests its importance since protein is derived from the Greek word prtos, meaning first.
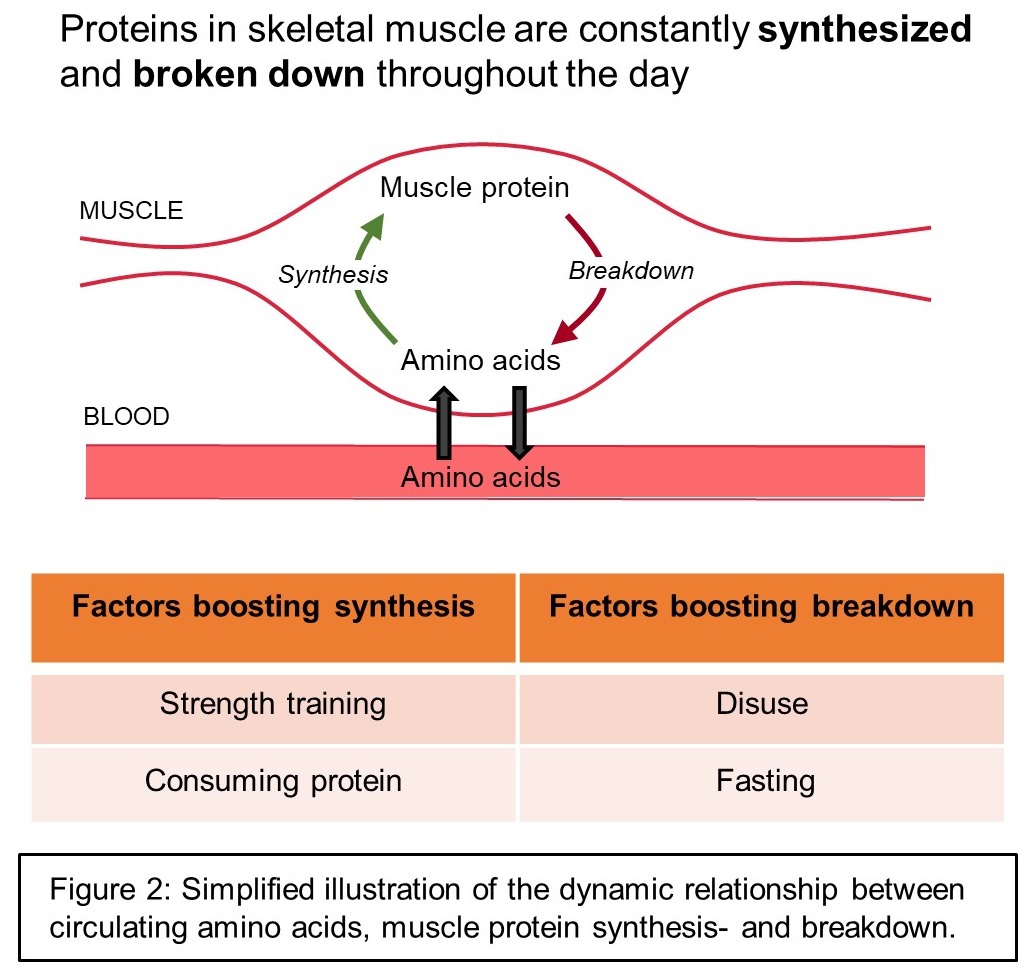
All cells, including muscle fibers, are in a constant process of making new proteins (synthesis of new proteins) and breaking old, dysfunctional proteins down (breakdown of proteins). The balance of these processes are frequently used as read-outs for the efficacy of various interventions (e.g. the source and timing of protein supplementation) in research studies.
These physiological processes are integral to skeletal muscle function and we want you to understand them particularly well, which is why we will start by digging a bit deeper into the fundamental biology underlying them.
1.2. Muscle protein synthesis
By Henning Langer and Gommaar DHulst
By far the most commonly used method to directly measure de novo protein synthesis is through the use of stable isotope labelled tracers. The basic concept is to flag a certain metabolite so that you can follow its fate in the body. A commonly used contemporary method is heavy water labeling with deuterium oxide (D O). Heavy water is not dangerous and completely identical to regular water, with the important exception that it contains deuterium instead of hydrogen. Deuterium is an isotope of hydrogen that has an additional neutron, which causes D O to literally contain heavier atoms than regular water:
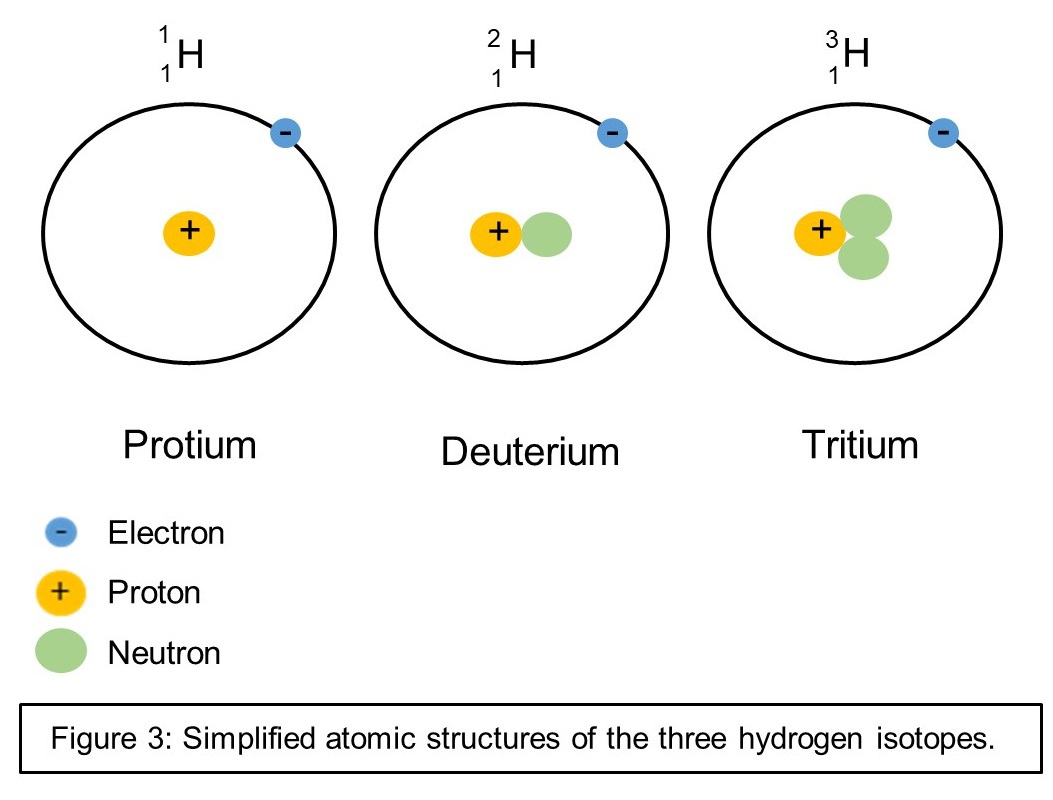
If somebody drinks heavy water, the main component of this water (i.e. deuterium) does everything that the components of regular water (i.e. regular hydrogen) would do. For example, a naturally occurring process is that certain amino acids can exchange one of their hydrogen atoms with circulating hydrogen. Alanine for example can replace up to four of its hydrogen atoms with fresh hydrogen. If alanine is exposed to deuterium, up to four of its hydrogen atoms can become replaced by deuterium. Since deuterium is heavier than regular hydrogen, alanine, which has incorporated deuterium into its molecular structure, is now heavier than regular alanine. This difference in molecular weight of alanine can be measured by different mass spectrometry techniques.
Like many other circulating amino acids, alanine is taken up by skeletal muscle and incorporated into newly made contractile proteins (i.e. de novo myofibrillar protein synthesis). By ingesting heavy water and labeling alanine in the circulation, we can trace the fate of alanine in different tissues. Imagine the following scenario:
Somebody drinks heavy water and his or her circulating alanine molecules become labeled with deuterium. If we now take two muscle tissue samples (biopsies) with two hours of time between them, we are able to tell how much new alanine got incorporated into the muscle samples by measuring how much heavier alanine in the muscle sample became over this time frame. If we also collect blood samples at the same time, we are then able to calculate how much heavier alanine became in the blood over those two hours. By comparing the rate at which alanine became heavier in the blood with the rate at which it became heav ier in muscle, we are then able to calculate the fractional synthetic rate (FSR) of proteins. There are different fractions in skeletal muscle (for example collagen or mitochondrial) and you will find different types of FSR measured in different papers, but for the purpose of this eBook we are referring to the de novo synthesis of contractile proteins, called myofibrillar protein synthesis. Whenever we mention MPS or FSR, we are referring to myofibrillar protein synthesis unless stated otherwise.