This book would not have been possible without the efforts of many people. First, I would like to thank Dr. William T. OBrien for paving the way with the original Top 3 book and leading me to this opportunity. Some cases from the nuclear medicine chapter of the second edition of the original work are included in this book. I would like to thank all those who trained me during my radiology residency at Penn State Health MS Hershey Medical Center and my nuclear medicine fellowship at the San Antonio Uniformed Services Health Education Consortium; it is because of you all that I am inspired to continue a career in academic radiology. Specific thanks to Dr. Don Fleming for taking a chance on meI am forever in your debt.
Many people contributed directly to this book. I would like to thank Joe Fotos, Mickaila Johnston, and Kate Wagner who were kind enough to serve as chapter editors. Thanks to all the residents and colleagues who authored or contributed to cases. I truly cannot thank any of them enough for helping make this project a reality!
Most importantly, I would like to thank my loving family! My wife, Lisa, has provided continuous love and support through the ups and downs, and many moves of my career. I am in awe of her strength daily. My daughters, Kiley and Makenzie, bring more joy to my life than I could have ever imagined! I have much to be grateful for!
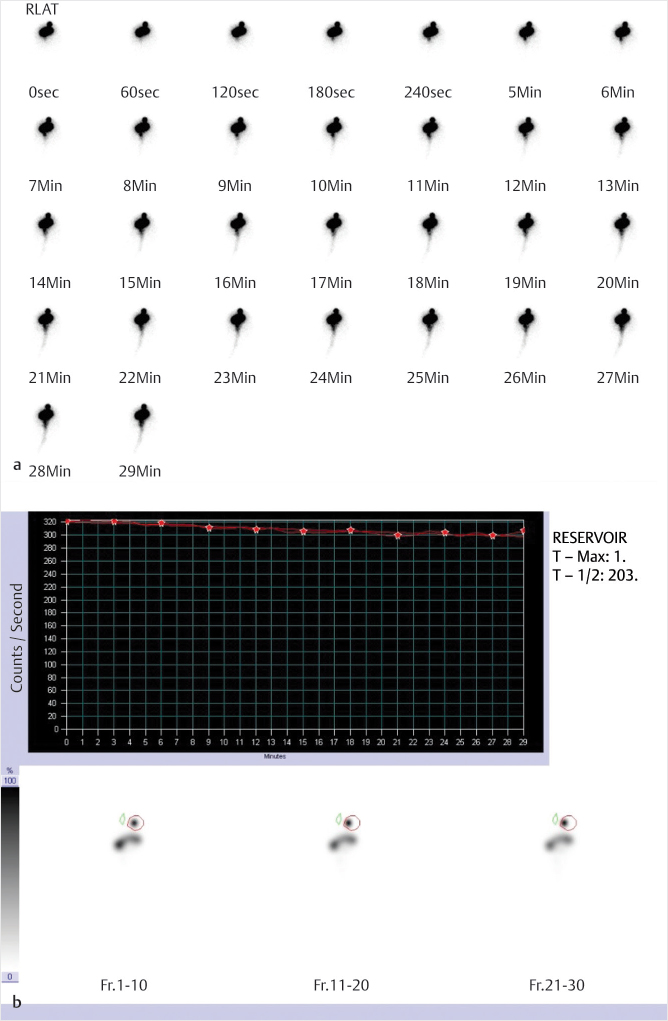
Fig. 1.1 Cerebrospinal fluid (CSF) flow study with indium-111 (In-111) diethylenetriamine pentaacetic acid (DTPA). Dynamic imaging (a) demonstrates activity localization within the reservoir and backflow into the ventricles and remaining neuraxis. Calculated T with a region of interest (ROI) at the reservoir (b) measures 203 minutes, markedly abnormal (normal is 5 minutes or less).
Clinical History
89-year-old male with history of idiopathic normal pressure hydrocephalus and worsening symptoms ().
Key Finding
Absent CSF flow through ventricular shunt tubing
Top 2 Differential Diagnoses
Shunt obstruction. Ventriculoperitoneal and ventriculoatrial shunts are commonly placed in patients with obstructive hydrocephalus. These shunts are placed percutaneously by neurosurgery. A reservoir, with an internal valve, is placed in the subcutaneous tissues superficial to the skull. Afferent tubing is then passed through the skull and into the ventricular system adjacent to the foramen of Monro. Efferent tubing is connected to the reservoir superficially and courses under the skin and down the neck and chest before terminating within the peritoneal cavity in the abdomen, or extending into the venous system and terminating in the right atrium. Worsening clinical symptoms or increasing ventricular size raises concern for obstruction of the shunt tubing. To evaluate the flow, the reservoir is accessed by the neurosurgical service and a small amount of the patients CSF is removed. The dose of In-111 DTPA is then injected into the reservoir in sterile saline, followed by a flush with the previously removed autologous CSF. The efferent tubing can be blocked if evaluation of the afferent tubing is needed. Dynamic imaging is performed for 30 minutes to visualize the flow of CSF. Quantification can be performed by drawing an ROI around the reservoir and measuring the time to half activity, normally 5 minutes or less. Normal examinations should demonstrate flow of activity from the lateral ventricles through the afferent tubing to the reservoir, and then through the efferent tubing to either the peritoneal or venous system. Complete obstruction will show flow of activity into the ventricles and absence of flow into the tubing or reservoir with no terminal destination activity. Possible etiologies for shunt obstruction include injury to the choroid plexus, increasing cellular debris within the CSF, as well as red blood cell and platelet aggregates in patients with intracranial hemorrhage.
Peritoneal pseudocyst. Normally, CSF that drains into the peritoneal cavity is absorbed over time. However, in rare cases a walled-off collection of the drained CSF can form within the peritoneal cavity. This collection is termed a peritoneal CSF pseudocyst which is formed by a wall of fibrous tissue and epithelium. As the cyst grows, backpressure through the peritoneal tubing increases leading to decreased and eventual absent forward flow. If the cyst is imaged early on in its development and growth, focal accumulation of activity within the abdomen is seen. If this diagnosis is suspected and no such accumulation is seen on CSF flow imaging, further imaging with abdominal ultrasound (US) or CT is essential for correct interpretation.
Diagnosis
CSF ventriculoperitoneal shunt tubing obstruction.
Pearls
Normal exams demonstrate flow from the ventricles to either the peritoneal cavity or venous system.
Shunt obstruction will present with absent or delayed antegrade flow.
Peritoneal CSF pseudocyst formation is a rare complication of VP shunt tubing placement.
Cross-sectional imaging is needed if CSF pseudocyst is suspected and flow imaging is negative.
In-111 DTPA is an ideal CSF label. It is nonpyrogenic with a favorable half-life and photon energy.
Suggested Readings
Khan F, Rehman A, Shamim MS, Bari ME. Factors affecting ventriculoperitoneal shunt survival in adult patients. Surg Neurol Int. 2015; 6:25
Mai DT, Vasinrapee P, Cook RE. Diagnosis of abdominal cerebrospinal fluid pseudocyst by scintigraphy. Clin Nucl Med. 1993; 18(3):237238
Sigg D, Rich R, Ashby S, Jabour B, Glass E. Radionuclide shuntogram demonstrating migration of distal ventriculoperitoneal shunt tubing out of the peritoneal cavity. Clin Nucl Med. 2005; 30(8):552554
Case 2
Kamal D. Singh
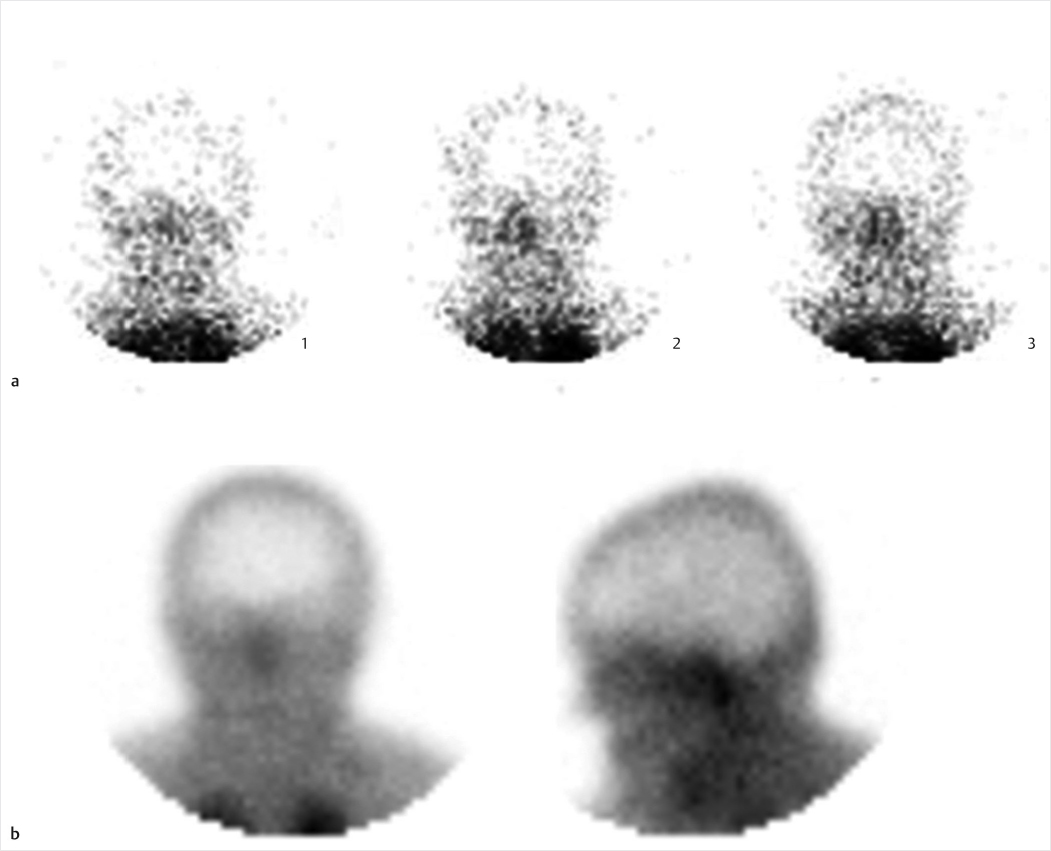
Fig. 2.1 Brain death study with Technetium-99m (Tc-99m) hexamethylpropyleneamine (HMPAO). Selected images from dynamic flow (1 second per frame for 1 minute) (a) demonstrate absence of intracranial flow. Blood pool imaging in anterior and lateral projections (b) reveals absent cerebral or cerebellar uptake and physiologic faint scalp activity, along with increased activity in the nasopharyngeal region (hot nose sign). These findings were confirmed on delayed single-photon emission computed tomography (SPECT) imaging of the head (not included).
Clinical History
57-year-old male unresponsive after a motor vehicle accident ().
Key Finding
Absent intracranial activity on a Tc-99m HMPAO scan
Top 3 Differential Diagnoses
Brain death. Brain death is a clinical diagnosis utilizing a combination of physical examination, electroencephalography, and imaging findings. A brain death scan has high specificity as a confirmatory study for absent intracranial perfusion. Scintigraphic imaging can be performed at the patients bedside with a portable gamma camera. Imaging may be performed with a nonspecific flow agent (Tc-99m diethylenetriamine pentaacetic acid [DTPA]), or with lipophilic brain perfusion agents (Tc-99m HMPAO or Tc-99m ethyl cysteinate dimer [ECD]) which cross the bloodbrain barrier (BBB) and are extracted by viable brain tissue proportional to cerebral flow. A scalp band or tourniquet may be placed around the head to avoid interfering activity from extracranial vessels. The scalp band, however, is contraindicated in pediatric patients due to increased intracranial pressure. First-minute dynamic flow imaging is performed in an anterior projection, with subsequent static blood pool images in both anterior and lateral projections. Delayed SPECT imaging may improve sensitivity in cases where lipophilic agents are utilized. Normal imaging reveals symmetric flow within the anterior and middle cerebral arteries (trident sign) on the anterior view, with visualization of dural venous sinuses on blood pool imaging. In brain death, there is termination of carotid activity at the skull base due to increased intracranial pressure overcoming perfusion pressure, thereby shunting blood through the external carotid artery branches projecting over the nasopharyngeal region (hot nose sign).