The main focus of this book is Heterogeneous Catalysis applied for (1) the elimination of atmospheric pollutants, (2) the production of clean energy, and (3) the valorization of chemical products in order to secure a more sustainable future. To efficiently implement these processes, it is essential to understand the intrinsic properties of the catalytic material and link it to its performance in electrocatalytic/catalytic reactions. This chapter will cover the basic principles of heterogeneous catalysis, including a brief historical, social, and economic overview and a discussion on the fundamentals of adsorption and surface reactivity, the methods of preparation of catalysts, and the catalytic properties. Understanding Heterogeneous Catalysis will reinforce the ability to relate theoretical concepts to practical issues that are addressed in detail in the following chapters.
Keywords
Heterogeneous catalysis; Pollutants; Clean energy; Chemical products; Adsorption; Surface; Reactivity; Catalysts; Preparation; Properties
Conflict of interest
The authors declare no conflict of interest.
1.1: Introduction
The development of the catalysis phenomenon has gone through several phases. In 1835, Jns Jakob Berzelius (17791848) suggested that the addition of small amounts of substances can affect transformations due to a catalytic force. Subsequently, in the early twentieth century, this concept has been modeled and associated with a kinetic nature. Wilhelm Oswald (18531932) introduced the concept of a catalyst as a substance capable of changing the rate of a chemical reaction without being consumed. Between the end of the nineteenth century and around 1920, Paul Sabatier (18541941) introduced a fundamental concept in developing catalysis in organic and inorganic synthesis: the formation of unstable intermediate compounds on the catalyst surface. The following period (until 1940) was marked by the development of the adsorption phenomenon. Hug Stott Taylor (18901974) suggested the existence of active sites on the surface of the catalyst. Irving Langmuir (18811957) presented his theory of adsorption, and in turn, Stephen Brunauer (19031986), Paul Hugh Emmett (19001985), and Edward Teller (19082003) formulated the BET equation to describe multilayer adsorption. Following this period, new interpretations about catalytic activity, new experimental techniques, and new catalytic processes have been developed ().
The use of catalysts for the most diverse purposes has intensified in the last decades since it is a powerful tool for controlling water and air pollutants and since it plays a vital role in many industrial processes. It is well known that catalysis plays an important role in the production of the vast majority of chemicals and other goods used by our society. It is estimated that 85%90% of all products manufactured in the chemical industry undergo catalytic processes ().
Many petroleum refineries use zeolite-based catalysts to break up long, complex, and diverse hydrocarbons present in crude oil into smaller fractions that can later be used as transportation fuels. Many chemicals, such as hydrogen, ammonia, urea, methanol, sulfuric acid, nitric acid, alcohols, ethylene oxide, ethylene glycol, ethylbenzene, and others, are mainly obtained through catalytic processes. Different catalysts have also been used for environmental protection, such as the abatement of CO, NO, hydrocarbons from automotive exhausts, and sulfur and nitrogen oxides from industrial exhaust streams (DeNOx/DeSOx technology) (). Besides, catalysts have also been used to capture and recover CO2 (main greenhouse gas) for its transformation into high value-added products.
This chapter is devoted to heterogeneous catalysis, which can be applied to several areas such as petrochemistry, environment, biomass, fine chemistry, etc. Clean energy and chemical production by the mean of heterogeneous catalysis are the main focus of this part as it is essential for the transition towards a more sustainable future. In this context, Heterogeneous Catalysis will be discussed in the scope of (1) the elimination of atmospheric pollutants, (2) the production of clean energy, and (3) the valorization of some molecules into value-added chemical products. To implement the aforementioned processes, it is essential to understand the properties of the catalytic materials and their role in the electrocatalytic/catalytic reactions, specifically the study of surface and interface chemistry.
This chapter is expected to become a must-read reference material to help in understanding the principles of heterogeneous catalysis (concepts and theories, physical adsorption, and chemical adsorption), the constitution of supported and/or promoted catalysts, and their properties (activity, selectivity, stability, and regenerability/deactivation). The study of the synthesis methods and the determination of several parameters (structural, microstructural, textural, surface acidity/basicity, reducibility, metallic dispersion, catalyst/support interactions, thermal, and mechanical) will also be highlighted whenever it is deemed crucial to the understanding of the catalytic performance in some specific reactions. Finally, this chapter will reinforce the ability to relate theoretical concepts to practical cases in the field of heterogeneous catalysis, with an emphasis on materials and their applications.
1.2: Catalysis fundamentals
Heterogeneous catalyst
Catalysis can be defined as a process of catalyst action. A catalyst increases the rate of a chemical reaction by providing a new and more comfortable pathway without modifying the thermodynamic factors.
The catalysis discipline is usually divided into heterogeneous, homogeneous, and enzymatic catalysis (or biocatalysis). In heterogeneous catalysis, also referred to as surface catalysis, the catalyst and the reactants are present in different phases. In general, the catalyst constitutes a solid phase while the reactants are in a fluid phase (solid-gas or solid-liquid). On the other hand, in homogeneous catalysis, the catalyst is generally an organometallic complex that is present with the reactant molecules in the same phase (liquid or gas). Finally, biocatalysis considers the use of enzymes to increase the rate of some types of chemical reactions.
This chapter will only consider heterogeneous catalysis. shows the principle of operation of a heterogeneous catalyst.
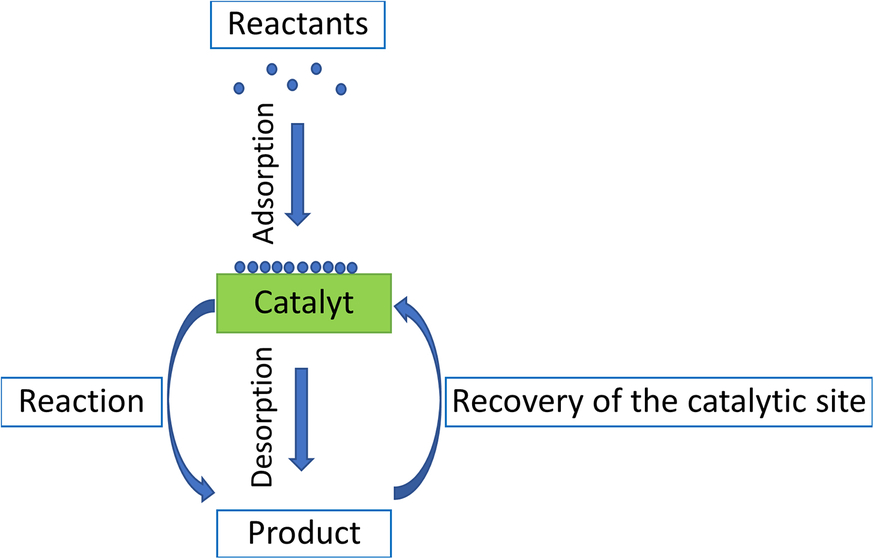
Elementary steps of the heterogeneous catalytic reaction.
This role of a heterogeneous catalyst can be described by the following cycle:
- Diffusion of the reactants to the catalyst;
- Adsorption of the reactants on the catalytic sites at the surface of the catalyst. The catalytic sites are specific locals on the surface of the solid where the reaction occurs (via heterogeneous catalysis);
- Chemical reaction between the adsorbed reactants molecules;
- Desorption of the products;
- Regeneration of the catalytic site for the new catalytic cycle.










 Elementary steps of the heterogeneous catalytic reaction.
Elementary steps of the heterogeneous catalytic reaction.