D. James Benton - Thermodynamics: Theory & Practice: The science of energy and power.
Here you can read online D. James Benton - Thermodynamics: Theory & Practice: The science of energy and power. full text of the book (entire story) in english for free. Download pdf and epub, get meaning, cover and reviews about this ebook. year: 2016, genre: Children. Description of the work, (preface) as well as reviews are available. Best literature library LitArk.com created for fans of good reading and offers a wide selection of genres:
Romance novel
Science fiction
Adventure
Detective
Science
History
Home and family
Prose
Art
Politics
Computer
Non-fiction
Religion
Business
Children
Humor
Choose a favorite category and find really read worthwhile books. Enjoy immersion in the world of imagination, feel the emotions of the characters or learn something new for yourself, make an fascinating discovery.
- Book:Thermodynamics: Theory & Practice: The science of energy and power.
- Author:
- Genre:
- Year:2016
- Rating:3 / 5
- Favourites:Add to favourites
- Your mark:
- 60
- 1
- 2
- 3
- 4
- 5
Thermodynamics: Theory & Practice: The science of energy and power.: summary, description and annotation
We offer to read an annotation, description, summary or preface (depends on what the author of the book "Thermodynamics: Theory & Practice: The science of energy and power." wrote himself). If you haven't found the necessary information about the book — write in the comments, we will try to find it.
D. James Benton: author's other books
Who wrote Thermodynamics: Theory & Practice: The science of energy and power.? Find out the surname, the name of the author of the book and a list of all author's works by series.
Thermodynamics: Theory & Practice: The science of energy and power. — read online for free the complete book (whole text) full work
Below is the text of the book, divided by pages. System saving the place of the last page read, allows you to conveniently read the book "Thermodynamics: Theory & Practice: The science of energy and power." online for free, without having to search again every time where you left off. Put a bookmark, and you can go to the page where you finished reading at any time.
Font size:
Interval:
Bookmark:
Thermodynamics:
Theory & Practice
The science of energy and power.
by D. James Benton
Copyright 2016 by D. James Benton, all rights reserved.
Foreword
...Thermodynamics is the branch of physical science that deals with energy in its various forms, including heat and work. Energy is a property of systems that can be stored and transferred to other systems. Energy is not the "ability to perform work." This is a misnomer. A system may contain considerable energy without having the capacity to perform any work. Thermodynamics is the key to understanding how things work. In this text we will explore classical (or macroscopic) as well as statistical (microscopic) thermodynamics, properties, processes, and heat engines. A variety of applications will be presented and all software is included.
...This book is not intended to be a textbook on thermodynamics or to replace any of the excellent textbooks that are already available. I hope you will find this to be a helpful companion to such texts. I cover several topics (e.g., microscopic point of view, speed distributions, and probability) that are never covered in textbooks on classical (i.e., macroscopic) thermodynamics and are often reserved for graduate courses. I consider these topics to be essential to understanding the whole of thermodynamics and many practical applications in particular. I hope this presentation will inspire you to dig deeper into this fascinating field.
All of the examples contained in this book,
(as well as a lot of free programs) are available at
http://www.dudleybenton.altervista.org/software/index.html
Table of Contents | page |
Foreword | i |
Chapter 1. Systems, Heat, and Work | |
Chapter 2. The First Law of Thermodynamics | |
Chapter 3. Microscopic vs. Macroscopic | |
Chapter 4. System of Particles | |
Chapter 5. Entropy & Probability | |
Chapter 6. The Second Law of Thermodynamics | |
Chapter 7. Free Energy & Maxwell's Relations | |
Chapter 8. Equations of State | |
Chapter 9. Saturation Properties | |
Chapter 10. Specific Heats | |
Chapter 11. Developing Thermodynamic Properties | |
Chapter 12. Carnot Cycle | |
Chapter 13. Steam Turbine Test | |
Chapter 14. Regenerative Rankine Cycle | |
Chapter 15. Gas Turbine Heat Balance | |
Chapter 16. Simple Combined Cycle | |
Chapter 17. Vapor-Compression Refrigeration Cycle | |
Chapter 18. Otto Cycle | |
Chapter 19. Diesel Cycle | |
Chapter 20. Brayton Cycle | |
Chapter 21. Lenoir Cycle | |
Chapter 22. Stirling Cycle | |
Chapter 23. Ericsson Cycle | |
Chapter 24. Throttling Natural Gas | |
Appendix A: A Microscopic Perspective on | |
Appendix B. van der Waals EOS Program | |
Appendix C. List of Refrigerants and Properties | |
Appendix D. Approximate Properties for Ideal Gases |
Chapter 1. Systems, Heat, and Work
...After energy, systems are the most important concept in thermodynamics and are essential even to understanding energy in its various forms. A system is an abstract group of one or more objects, separated from everything else by a boundary. Although a system boundary may correspond to a physical boundary, such as a tank, this is not a requirement. A system boundary has no mass and occupies no space (i.e., has no thickness).
...Furthermore, a system boundary may move, objects and energy may pass through it, and it may not be physically possible to construct a corresponding physical boundary. An example of this would be the oxygen molecules in a room. It is not possibleand may not even be desirableto separate the oxygen molecules from the nitrogen and other gases in the room, but this doesn't preclude us from considering a system containing only the oxygen molecules.
...Even in the case of something as simple as a tank, it may be advantageous to consider the contents and the tank separately. We can imagine any shape or form of system boundary and even multiple overlapping system boundaries. There are an infinite number of such selections. All selections for a system boundary are not equal. Some selections are far more useful than others. There is quite an art to the selection of a system boundary. Once we have selected a system boundary, it is essential that we handle it properly and consistently.
...There are three basic types of system boundaries: 1) open, 2) closed, and 3) isolated. An open system can exchange mass and energy with its surroundings. A closed system can exchange energy, but not mass with its surroundings. An isolated system cannot exchange mass or energy with its surroundings. Closed and isolated systems are often confused and there is much useless debate over this confusion.[1]
...Heat is that transient form of energy that crosses a system boundary by virtue of a temperature gradient. Work is that transient form of energy that crosses a system boundary by virtue of a force. Systems do not contain heat or work and neither can be stored. Only energy is stored. All other forms of energy that cross a system boundary apart from mass can be classified as either heat or work. For example, electricity is a form of work. Electrons flow because of a force, not a temperature. Systems do not contain temperature. Temperaturewhen applicablemay be a measure of the energy of a system.
...Because energy is a property of objectsthat is, objects can contain energywhen mass crosses a system boundary (i.e., an open system), energy is transferred from the system to its surroundings (outflow) or from the surroundings to a system (inflow). It is essential to recognize that the surroundings include everything that isn't inside the system boundary. For convenience, the surroundings may be separated into the immediate and the distant or ultimate. The three types of systems are illustrated in the following figure:
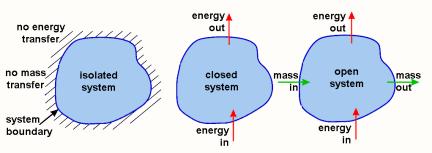
...For an ideal closed system (e.g., a cylinder fitted with a frictionless piston that doesn't leak), the work is equal to the integral of force with respect to distance. For a piston, force is equal to pressure times area, so that:
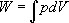
(1.1)
...The work per unit mass (i.e., W/m ) is given the symbol w . Specific volume is defined as volume per unit mass (i.e., V/m ) and is given the symbol v . These two definitions can be substituted into Equation 1.1 to obtain:
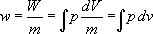
(1.2)
...For a continuously flowing open system, power is equal to the integral of force times velocity or pressure differential times volumetric flow rate. The volumetric flow rate is equal to the specific volume times the mass flow rate; thus we have the following equation for the ideal power of a continuously flowing open system:
Next pageFont size:
Interval:
Bookmark:
Similar books «Thermodynamics: Theory & Practice: The science of energy and power.»
Look at similar books to Thermodynamics: Theory & Practice: The science of energy and power.. We have selected literature similar in name and meaning in the hope of providing readers with more options to find new, interesting, not yet read works.
Discussion, reviews of the book Thermodynamics: Theory & Practice: The science of energy and power. and just readers' own opinions. Leave your comments, write what you think about the work, its meaning or the main characters. Specify what exactly you liked and what you didn't like, and why you think so.








