Wong - Mechanism and Theory in Food Chemistry
Here you can read online Wong - Mechanism and Theory in Food Chemistry full text of the book (entire story) in english for free. Download pdf and epub, get meaning, cover and reviews about this ebook. City: Cham, year: 2018, publisher: Springer International Publishing, genre: Children. Description of the work, (preface) as well as reviews are available. Best literature library LitArk.com created for fans of good reading and offers a wide selection of genres:
Romance novel
Science fiction
Adventure
Detective
Science
History
Home and family
Prose
Art
Politics
Computer
Non-fiction
Religion
Business
Children
Humor
Choose a favorite category and find really read worthwhile books. Enjoy immersion in the world of imagination, feel the emotions of the characters or learn something new for yourself, make an fascinating discovery.
- Book:Mechanism and Theory in Food Chemistry
- Author:
- Publisher:Springer International Publishing
- Genre:
- Year:2018
- City:Cham
- Rating:5 / 5
- Favourites:Add to favourites
- Your mark:
- 100
- 1
- 2
- 3
- 4
- 5
Mechanism and Theory in Food Chemistry: summary, description and annotation
We offer to read an annotation, description, summary or preface (depends on what the author of the book "Mechanism and Theory in Food Chemistry" wrote himself). If you haven't found the necessary information about the book — write in the comments, we will try to find it.
Wong: author's other books
Who wrote Mechanism and Theory in Food Chemistry? Find out the surname, the name of the author of the book and a list of all author's works by series.
Mechanism and Theory in Food Chemistry — read online for free the complete book (whole text) full work
Below is the text of the book, divided by pages. System saving the place of the last page read, allows you to conveniently read the book "Mechanism and Theory in Food Chemistry" online for free, without having to search again every time where you left off. Put a bookmark, and you can go to the page where you finished reading at any time.
Font size:
Interval:
Bookmark:
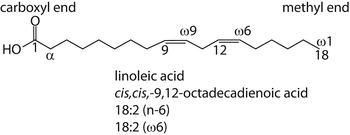 | |||
Chain length | Common name | Systemic name | |
Saturated | 10:0 | Capric acid | Decanoic |
12:0 | Lauric acid | Dodecanoic | |
14:0 | Myristic acid | Tetradecanoic | |
16:0 | Palmitic acid | Hexadecanoic | |
18:0 | Stearic acid | Octadecanoic | |
20:0 | Arachidic acid | Eicosanoic | |
22:0 | Behenic acid | Docosanoic | |
Mono-unsaturated | 16:1 (n-7) | Palmitoleic acid | cis-9-Hexadecenoic |
18:1 (n-9) | Oleic acid | cis -9-Octadecenoic | |
22:1 (n-9) | Erucic acid | cis -10-Docosenoic | |
Di-unsaturated | 18:2 (n-6) | Linoleic acid | cis,cis -9 , 12-Octadecadienoic |
Tri-unsaturated | 18:3 (n-6) | -linolenic acid | cis,cis , cis -6,9 , 12-Octadecatrienoic acid |
18:3 (n-3) | -linolenic acid | cis,cis,cis- 9,12,15-Octadecatrienoic acid | |
20:4 (n-6) | Arachidonic | 5,8,11,14-Eicosatetraenoic acid (all cis) | |
20:5 (n-3) | EPA | 5,8,11,14,17-Eicosapentaenoic acid (all cis) | |
20:6 (n-3) | DHA | 4,7,10,13,16,19-Docosahexaenoic (all cis) |
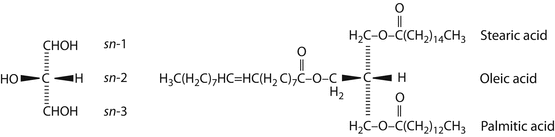
Font size:
Interval:
Bookmark:
Similar books «Mechanism and Theory in Food Chemistry»
Look at similar books to Mechanism and Theory in Food Chemistry. We have selected literature similar in name and meaning in the hope of providing readers with more options to find new, interesting, not yet read works.
Discussion, reviews of the book Mechanism and Theory in Food Chemistry and just readers' own opinions. Leave your comments, write what you think about the work, its meaning or the main characters. Specify what exactly you liked and what you didn't like, and why you think so.













