Georg F. Hoffmann , Johannes Zschocke and William L. Nyhan (eds.) Inherited Metabolic Diseases A Clinical Approach 10.1007/978-3-540-74723-9_1 Springer-Verlag Berlin Heidelberg 2010
1. Disorders of Intermediary Metabolism
Johannes Zschocke 1
(1)
Divisions of Human Genetics and Clinical Genetics, Medical University Innsbruck, Schpfstr. 41, Innsbruck, 6020, Austria
Johannes Zschocke Professor of Human Genetics
Email:
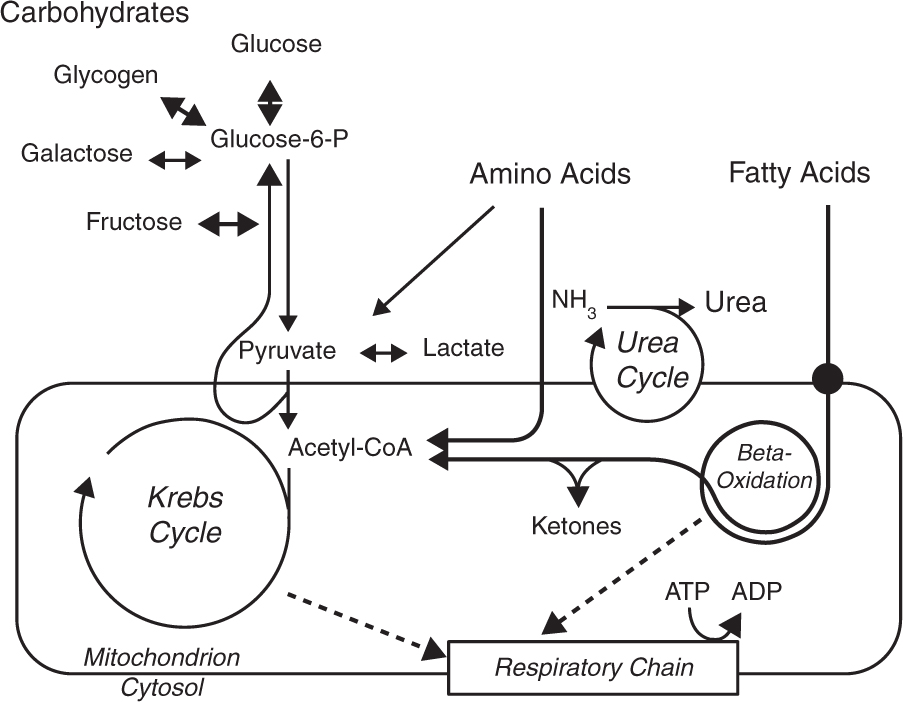
The classical inborn errors of metabolism are defects in enzymes of the metabolism of amino acids, carbohydrates, and fatty acids or in mitochondrial energy metabolism (Fig. ). These disorders are often dynamic; they fluctuate with changes in the metabolic state of the patient and frequently allow successful therapeutic intervention. Most of them are readily diagnosed through basic metabolic investigations, which include blood gases, glucose, lactate, ammonia, plasma amino acids, urinary organic acids, and an acylcarnitine profile.
Fig. A1.1
Main pathways of intermediary metabolism
1.1 Disorders of Amino Acido Metabolism
Typical aminoacidopathies result from abnormalities in the breakdown of amino acids in the cytosol. In addition, several disorders involving mitochondrial enzymes such as branched-chain ketoacid dehydrogenase (maple syrup urine disease) or ornithine aminotransferase (gyrate atrophy of the choroidea) are classified as amin-oacidopathies as they do not involve CoA-activated metabolites. This distinguishes aminoacidopathies from the organic acidurias, which are considered a separate group of disorders affecting mitochondrial enzymes, CoA-activated metabolites, and which have effects on other mitochondrial functions. Clinical symptoms of the aminoacidopathies may be thought of as caused by the accumulation of toxic intermediates that cause specific organ damage. Several defects of amino acid metabolism such as histidinemia are benign because the metabolites that accumulate are not toxic. The pathogenetic relevance of an inborn error of amino acid metabolism is not always easy to ascertain as clinical symptoms observed in the child may be coincidental or the reason for performing the analysis in the first place. Aminoacidopathies are diagnosed through the analysis of plasma (or urinary) concentrations of amino acids and sometimes of urinary organic acids. A majority is treatable through dietary restriction of protein and of the amino acid involved in the defective pathway and by the avoidance or prompt treatment of catabolic states that lead to the breakdown of large amounts of protein. Another therapeutic strategy that has been successful in hepatorenal tyrosinemia is the inhibition of a biochemical step before the actual genetic deficiency, thereby changing a harmful disease into a more benign amino acid accumulation without the accumulation of the more damaging substances downstream.
1.2 Organic Acidurias
The classical organic acidurias are deficiencies of enzymes in the mitochondrial metabolism of CoA-activated carboxylic acids, most of which are derived from amino acid breakdown. In this way, they are distinguished from disorders of fatty acid oxidation, which not only involve CoA esters but also present different diagnostic and therapeutic challenges. The term organic acidurias is preferred to the alternative term organic acidemias as they are most often detected by analysis of the urine. Biochemically, some of the reactions impaired in the organic acidurias are parallel to the dehydrogenase, hydratase, or ketothiolase reactions of the mitochondrial -oxidation cycle. Clinical features are caused not only by the accumulation of toxic intermediates but also by a disturbance of mito-chondrial energy metabolism and carnitine homeosta-sis; they may include encephalopathy and episodic metabolic acidosis. Organic acidurias are diagnosed through the analysis of organic acids in the urine or acylcarnitines in the blood. Treatment is similar to that of the aminoacidopathies and involves the dietary restriction of the relevant amino acid(s) and the avoidance of protein catabolism. However, as the defective enzymes are distant (more downstream) from the respective amino acids, restriction may not lead to a stoichiometric reduction of pathological metabolites, although it does in methylmalonic aciduria. Unexpected fluctuations occur and complete return to normal intermediary metabolism is usually impossible. Supplementation with carnitine and sometimes other substances such as glycine (e.g., to form isovalerylglycine in isovaleric aciduria) are very useful adjuncts to the treatment.
Disorders of biotin metabolism are included among the organic acidurias. Biotin is a cofactor of the mito-chondrial carboxylases and a deficiency of biotini-dase or holocarboxylase synthetase leads to multiple carboxylase deficiency. It is also usually diagnosed through urinary organic acid analysis. Biotinidase enzyme analysis of dried blood spots has been included into programs of neonatal screening as it is well treated with biotin supplementation.
1.3 Disorders of Ammonia Detoxification
The breakdown of protein produces large amounts of nitrogen in the form of ammonia that is highly neurotoxic but is normally converted to urea and excreted in the urine. Defects in enzymes of the urea cycle and other disorders of ammonia detoxification present clinically with encephalopathy and other symptoms of hyperammonemia. Metabolic investigations should include analysis of the amino acids in plasma and urine and urinary orotic acid. Treatment requires the reduction of protein intake in conjunction with the supplementation of essential amino acids, the avoidance of catabolic states and the administration of benzoate or phenylacetate/phe-nylbutyrate, which remove nitrogen in the form of alternative conjugates of amino acids such as glycine and glutamine.
1.4 Disorders of Fatty Acid Oxidation and Ketogenesis
Mitochondrial fatty acid oxidation is required for the provision of energy during fasting, either through complete oxidation or through production of ketones in the liver that then serve as an alternative energy source for the brain. Disorders in this pathway typically present as hypoketotic hypoglycemia precipitated by fasting, leading to coma or convulsions. In addition, some disorders cause severe hepatopathy and (cardio-) myopathy, probably as results of the accumulation of toxic metabolites. The diagnosis is best reached in the acute situation through the analysis of free fatty acids and the ketone bodies 3-hydroxybutyrate and acetoac-etate as well as the acylcarnitine profile and urinary organic acids. The diagnosis may be missed if samples are obtained in the normal interval between episodes or after the patient has been treated with intravenous glucose. Treatment consists of avoidance of fasting. Carnitine supplementation is mostly unessessary and must be carefully balanced in some defects, particularly those that cause cardiomyopathy or hepatopathy.