For my husband, who poured our first density rainbow with steady hands.
For my children, a.k.a. lab assistants, who joined me in smashing, melting, dissolving, smelling, braiding, stretching, and destroying candy for the sake of science.
And especially for my oldest daughter, who put her Nerds in water and invented candy experiments.
CANDY EXPERIMENTS
copyright 2012 by Loralee Leavitt. All rights reserved.
No part of this book may be used or reproduced
in any manner whatsoever without written permission except in the case
of reprints in the context of reviews.
Andrews McMeel Publishing, LLC
an Andrews McMeel Universal company
1130 Walnut Street, Kansas City, Missouri 64106
www.andrewsmcmeel.com
ISBN: 978-1-4494-1837-3
Library of Congress Control Number: 2011944678
Design by Holly Ogden
All experiments in this book should be performed with adult supervision by parents or guardians. Neither the author nor the publisher assumes any responsibility for any injuries or damages arising from any activities.
Attention: Schools and Businesses
Andrews McMeel books are available at quantity discounts with bulk purchase for educational, business, or sales promotional use. For information, please e-mail the Andrews McMeel Publishing Special Sales Department:
CONTENTS
c h a p te r
SECRET
INGREDIENTS
Candy is made from sugar. But it also has secret ingredients that give it flavor and texture.
Heres how to test for some of them.
Time: 1 to 5 minutes
Skill Level: Easy
Acid in your stomach helps you digest your food. Acid on your teeth breaks down the enamel and causes tooth decay. Acid in lemon juice makes it taste sour . Is there acid in your candy ?
A sour taste is the bodys way of identifying acid. If your candy tastes sour, it contains acid. To test for acid, try this.
What you need:
Sour candy, such as Warheads , Lemonheads , Sour Skittles , sour Laffy Taffy , Sour Patch Kids or other sour gummies , or Pixy Stix
Small bowl filled with cup warm water
Baking soda
What to do:
Drop the candy into the water. Let the sour part of the candy dissolve, such as the sour powder on gummies or the outer coating of Lemonheads. You can stir the water to help the candy dissolve faster. (If a waxy layer forms on the surface of the water, try to scrape or spoon it off.)
Sprinkle a spoonful of baking soda into the water.
Watch for bubbles . If bubbles form, theres acid in the candy.
Alternative:
For super-sour candy like Warheads, start by dissolving the baking soda in the water. Then drop in the Warheads and watch the bubbles rise.
Whats happening:
When baking soda (a base) mixes with an acid, it releases carbon dioxide gas. This is what forms the bubbles. The more bubbles you see, the more acidic the candy is.
This same reaction is what makes bubbles in baked foods like crackers, cookies, and pancakes. In some food, baking soda reacts with acid in the dough. Cooks also use baking powder, a mixture of baking soda and acid that bubbles when its wet.
Bubbles rise from acidic Warheads dropped in baking soda water.
m o r e f u n
Ever tried a fizzy Zotz candy ? This kind of candy contains its own acid test. In the candy filling, acid is mixed with baking soda. When the filling gets wet, the acid reacts with the baking soda, creating fizzy bubbles.
To see Zotz in action, drop a piece into some water and watch for bubbles. For faster results, smash it first.
CAUTION: Dont eat lots of baking soda or drink lots of baking soda water. It could make your body too basic (the opposite of acidic), and you might get sick.
Time: 5 minutes (plus 1 hour to make indicator)
Skill Level: Medium
You can use color, such as the color in purple cabbage , to detect the acid in sour candy . To see the color change, try this.
What you need:
Small bowls
Purple cabbage indicator (To make this, rip or chop enough purple cabbage to fill about 1 cup and soak it in 1 cup of hot water until the water is purple. For a darker color, boil the cabbage in water.)
Sour candy, such as Warheads , Lemonheads , SweeTARTS , or sour gummies (avoid red or pink)
What to do:
Fill a bowl with the cabbage indicator.
Add a piece of the candy and let it dissolve. You can stir the water to help the candy dissolve faster.
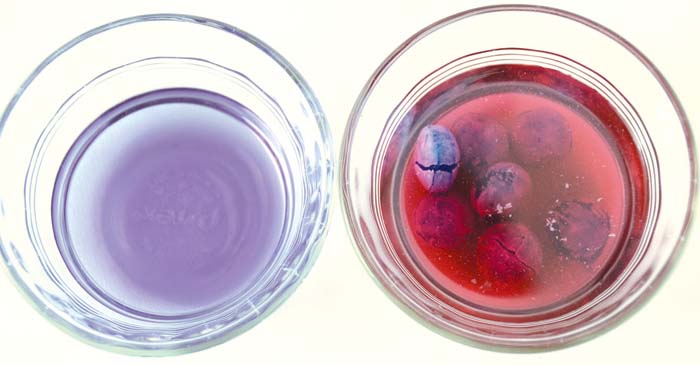
If the indicator turns pink, the candy is acidic.
Whats happening:
The pigment in purple cabbage turns pink in acid. When you dissolve acidic candy, the acid changes the cabbage color to pink. (If you used red or pink candy for the experiment , you might not be able to tell if the cabbage color actually changed.)
Many kinds of candy contain acid, especially if they taste sour, like Lemonheads, Skittles , Starburst , and Swe e T ARTS . To find more acidic candy, check the labels for ingredients like citric acid or malic acid, or try this test with candy that tastes sour.
Acidic Warheads turn purple cabbage indicator pink.
m o r e f u n
The cabbage indicator turns blue when you add a base . To make your candy water basic, add baking soda and watch the color change.
You can also use your purple cabbage indicator to test other household ingredients for acid, like vinegar, lemon juice, or clear soda, such as 7UP. If you have any indicator left, store it in an airtight container in the refrigerator.
Time: 5 to 10 minutes
Skill Level: Easy
Many chewy candies contain a different secret ingredient: oil . Can you see the oil for yourself?
What you need:
Chewy candy containing oil, such as Starburst , Skittles , Tootsie Rolls , or taffy
Small bowl of warm water
What to do:
Drop the candy into the bowl of warm water. Let it start to dissolve.
After a few minutes, look for shiny puddles floating on the surface.
When the water cools, look for a white waxy layer on top.
Whats happening:
Many kinds of chewy candy are made with oil, such as hydrogenated palm kernel oil. The oil keeps the candy from sticking to the machinery in the candy factory. It also helps make the candy smooth, soft, and chewy.
When you dissolve the candy in water, the hydrogenated palm kernel oil melts and forms the shiny puddles. In colder water, it can cool to a white, waxy solid. Since oil is lighter than water, it floats.